当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Cycle of Glycoside Hydrolase BglX from Pseudomonas aeruginosa and Its Implications for Biofilm Formation.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-01-07 , DOI: 10.1021/acschembio.9b00754 Kiran V Mahasenan 1 , María T Batuecas 2 , Stefania De Benedetti 1 , Choon Kim 1 , Neha Rana 1 , Mijoon Lee 1 , Dusan Hesek 1 , Jed F Fisher 1 , Julia Sanz-Aparicio 2 , Juan A Hermoso 2 , Shahriar Mobashery 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-01-07 , DOI: 10.1021/acschembio.9b00754 Kiran V Mahasenan 1 , María T Batuecas 2 , Stefania De Benedetti 1 , Choon Kim 1 , Neha Rana 1 , Mijoon Lee 1 , Dusan Hesek 1 , Jed F Fisher 1 , Julia Sanz-Aparicio 2 , Juan A Hermoso 2 , Shahriar Mobashery 1
Affiliation
|
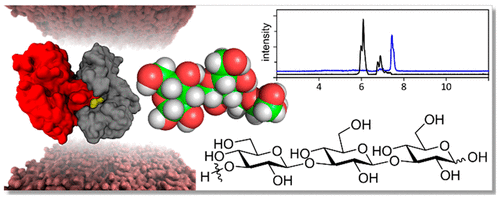
BglX is a heretofore uncharacterized periplasmic glycoside hydrolase (GH) of the human pathogen Pseudomonas aeruginosa. X-ray analysis identifies it as a protein homodimer. The two active sites of the homodimer comprise catalytic residues provided by each monomer. This arrangement is seen in <2% of the hydrolases of known structure. In vitro substrate profiling shows BglX is a catalyst for β-(1→2) and β-(1→3) saccharide hydrolysis. Saccharides with β-(1→4) or β-(1→6) bonds, and the β-(1→4) muropeptides from the cell-wall peptidoglycan, are not substrates. Additional structural insights from X-ray analysis (including structures of a mutant enzyme-derived Michaelis complex, two transition-state mimetics, and two enzyme-product complexes) enabled the comprehensive description of BglX catalysis. The half-chair (4H3) conformation of the transition-state oxocarbenium species, the approach of the hydrolytic water molecule to the oxocarbenium species, and the stepwise release of the two reaction products were also visualized. The substrate pattern for BglX aligns with the [β-(1→2)-Glc]x and [β-(1→3)-Glc]x periplasmic osmoregulated periplasmic glucans, and possibly with the Psl exopolysaccharides, of P. aeruginosa. Both polysaccharides are implicated in biofilm formation. Accordingly, we show that inactivation of the bglX gene of P. aeruginosa PAO1 attenuates biofilm formation.
中文翻译:

铜绿假单胞菌糖苷水解酶 BglX 的催化循环及其对生物膜形成的影响。
BglX 是迄今为止尚未表征的人类病原体铜绿假单胞菌的周质糖苷水解酶 (GH)。X 射线分析将其鉴定为蛋白质同二聚体。同型二聚体的两个活性位点包含由每个单体提供的催化残基。这种排列在 <2% 的已知结构的水解酶中可见。体外底物分析显示 BglX 是 β-(1→2) 和 β-(1→3) 糖水解的催化剂。具有 β-(1→4) 或 β-(1→6) 键的糖类以及来自细胞壁肽聚糖的 β-(1→4) 胞肽不是底物。X 射线分析的其他结构见解(包括突变酶衍生的米氏复合物、两种过渡态模拟物和两种酶产物复合物的结构)使得能够全面描述 BglX 催化作用。过渡态氧碳鎓物种的半椅(4H3)构象、水解水分子接近氧碳鎓物种以及两种反应产物的逐步释放也被可视化。BglX 的底物模式与 [β-(1→2)-Glc]x 和 [β-(1→3)-Glc]x 周质渗透调节周质葡聚糖一致,并且可能与铜绿假单胞菌的 Psl 胞外多糖一致。两种多糖都与生物膜的形成有关。因此,我们表明铜绿假单胞菌 PAO1 的 bglX 基因失活会减弱生物膜的形成。
更新日期:2020-01-07
中文翻译:

铜绿假单胞菌糖苷水解酶 BglX 的催化循环及其对生物膜形成的影响。
BglX 是迄今为止尚未表征的人类病原体铜绿假单胞菌的周质糖苷水解酶 (GH)。X 射线分析将其鉴定为蛋白质同二聚体。同型二聚体的两个活性位点包含由每个单体提供的催化残基。这种排列在 <2% 的已知结构的水解酶中可见。体外底物分析显示 BglX 是 β-(1→2) 和 β-(1→3) 糖水解的催化剂。具有 β-(1→4) 或 β-(1→6) 键的糖类以及来自细胞壁肽聚糖的 β-(1→4) 胞肽不是底物。X 射线分析的其他结构见解(包括突变酶衍生的米氏复合物、两种过渡态模拟物和两种酶产物复合物的结构)使得能够全面描述 BglX 催化作用。过渡态氧碳鎓物种的半椅(4H3)构象、水解水分子接近氧碳鎓物种以及两种反应产物的逐步释放也被可视化。BglX 的底物模式与 [β-(1→2)-Glc]x 和 [β-(1→3)-Glc]x 周质渗透调节周质葡聚糖一致,并且可能与铜绿假单胞菌的 Psl 胞外多糖一致。两种多糖都与生物膜的形成有关。因此,我们表明铜绿假单胞菌 PAO1 的 bglX 基因失活会减弱生物膜的形成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号