JAMA Oncology ( IF 28.4 ) Pub Date : 2019-12-26 , DOI: 10.1001/jamaoncol.2019.5566 Thomas M Atkinson 1 , Amylou C Dueck 2 , Daniel V Satele 3 , Gita Thanarajasingam 4 , Jacqueline M Lafky 4 , Jeff A Sloan 3 , Ethan Basch 1, 5
|
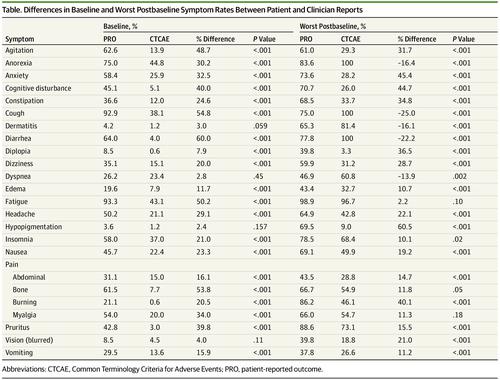
Many patients enter cancer clinical trials with baseline symptoms.1 Notably, the current clinician reporting mechanism for symptomatic adverse events (AEs) via the Common Terminology Criteria for Adverse Events (CTCAE)2 does not formally distinguish between symptoms present at baseline vs those that develop during a trial. Therefore, AE estimation in clinical trials may include symptoms that predate trial entry. This raises concern that the cumulative incidence of patient-reported AEs may be high, particularly if preexisting symptoms related to other causes (eg, comorbidities, prior treatment) are attributed to study drugs.
中文翻译:

癌症临床试验中不良事件评估的基线和基线后症状的临床医生与患者报告。
许多患者以基线症状进入癌症临床试验。1值得注意的是,目前的临床不良反应症状报告(AEs)通过不良事件通用术语标准(CTCAE)2的临床报告机制并未正式地区分基线出现的症状和试验期间出现的症状。因此,临床试验中的AE估算可能包括在试验进入之前的症状。这引起人们的担忧,即患者报告的AE的累积发生率可能很高,特别是如果与其他原因(例如合并症,在先治疗)相关的先前症状归因于研究药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号