Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A GPR174–CCL21 module imparts sexual dimorphism to humoral immunity
Nature ( IF 64.8 ) Pub Date : 2019-12-25 , DOI: 10.1038/s41586-019-1873-0 Ruozhu Zhao 1, 2, 3, 4 , Xin Chen 1, 2, 3, 4 , Weiwei Ma 1, 2, 3 , Jinyu Zhang 2, 3, 5 , Jie Guo 6 , Xiu Zhong 4 , Jiacheng Yao 4 , Jiahui Sun 1, 2, 3 , Julian Rubinfien 2 , Xuyu Zhou 6 , Jianbin Wang 4 , Hai Qi 1, 2, 3, 4, 7, 8
Nature ( IF 64.8 ) Pub Date : 2019-12-25 , DOI: 10.1038/s41586-019-1873-0 Ruozhu Zhao 1, 2, 3, 4 , Xin Chen 1, 2, 3, 4 , Weiwei Ma 1, 2, 3 , Jinyu Zhang 2, 3, 5 , Jie Guo 6 , Xiu Zhong 4 , Jiacheng Yao 4 , Jiahui Sun 1, 2, 3 , Julian Rubinfien 2 , Xuyu Zhou 6 , Jianbin Wang 4 , Hai Qi 1, 2, 3, 4, 7, 8
Affiliation
|
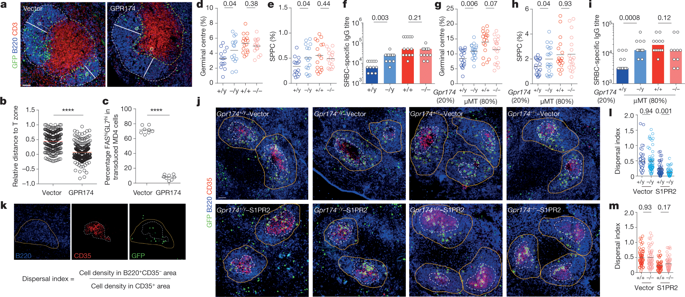
Humoral immune responses to immunization and infection and susceptibilities to antibody-mediated autoimmunity are generally lower in males 1 – 3 . However, the mechanisms underlying such sexual dimorphism are not well understood. Here we show that there are intrinsic differences between the B cells that produce germinal centres in male and female mice. We find that antigen-activated male B cells do not position themselves as efficiently as female B cells in the centre of follicles in secondary lymphoid organs, in which germinal centres normally develop. Moreover, GPR174—an X-chromosome-encoded G-protein-coupled receptor—suppresses the formation of germinal centres in male, but not female, mice. This effect is intrinsic to B cells, and correlates with the GPR174-enhanced positioning of B cells towards the T-cell–B-cell border of follicles, and the distraction of male, but not female, B cells from S1PR2-driven follicle-centre localization. Biochemical fractionation of conditioned media that induce B-cell migration in a GPR174-dependent manner identifies CCL21 as a GPR174 ligand. In response to CCL21, GPR174 triggers a calcium flux and preferentially induces the migration of male B cells; GPR174 also becomes associated with more Gαi protein in male than in female B cells. Male B cells from orchidectomized mice exhibit impaired GPR174-mediated migration to CCL21, and testosterone treatment rescues this defect. Female B cells from testosterone-treated mice exhibit male-like GPR174–Gαi association and GPR174-mediated migration. Deleting GPR174 from male B cells causes more efficient positioning towards the follicular centre, the formation of more germinal centres and an increased susceptibility to B-cell-dependent experimental autoimmune encephalomyelitis. By identifying GPR174 as a receptor for CCL21 and demonstrating its sex-dependent control of B-cell positioning and participation in germinal centres, we have revealed a mechanism by which B-cell physiology is fine-tuned to impart sexual dimorphism to humoral immunity. Male and female B cells show differing abilities to localize and contribute to germinal centres, in a way that depends on the G-protein-coupled guidance receptor GPR174 and its chemokine ligand CCL21.
中文翻译:

GPR174-CCL21 模块赋予体液免疫性二态性
男性对免疫和感染的体液免疫反应以及对抗体介导的自身免疫的易感性通常较低 1 – 3 。然而,这种性别二态性的潜在机制尚不清楚。在这里,我们表明在雄性和雌性小鼠中产生生发中心的 B 细胞之间存在内在差异。我们发现抗原激活的雄性 B 细胞不能像雌性 B 细胞那样有效地将自身定位在次级淋巴器官的卵泡中心,在这些器官中,生发中心通常会发育。此外,GPR174——一种 X 染色体编码的 G 蛋白偶联受体——抑制雄性小鼠而不是雌性小鼠的生发中心的形成。这种效应是 B 细胞固有的,并且与 GPR174 增强的 B 细胞定位于毛囊的 T 细胞-B 细胞边界相关,以及来自 S1PR2 驱动的卵泡中心定位的男性而非女性 B 细胞的注意力分散。以 GPR174 依赖性方式诱导 B 细胞迁移的条件培养基的生化分级将 CCL21 鉴定为 GPR174 配体。响应 CCL21,GPR174 触发钙通量并优先诱导雄性 B 细胞的迁移;与女性 B 细胞相比,GPR174 还与男性中更多的 Gαi 蛋白相关。来自兰花切除小鼠的雄性 B 细胞表现出 GPR174 介导的 CCL21 迁移受损,而睾酮治疗可以挽救这种缺陷。来自睾酮处理小鼠的雌性 B 细胞表现出类似雄性的 GPR174-Gαi 关联和 GPR174 介导的迁移。从雄性 B 细胞中删除 GPR174 会更有效地定位到卵泡中心,更多生发中心的形成和对 B 细胞依赖性实验性自身免疫性脑脊髓炎的易感性增加。通过将 GPR174 鉴定为 CCL21 的受体并证明其对 B 细胞定位和参与生发中心的性别依赖性控制,我们揭示了一种机制,通过该机制可以微调 B 细胞生理学以赋予体液免疫性二态性。男性和女性 B 细胞显示出不同的定位和促进生发中心的能力,其方式取决于 G 蛋白偶联引导受体 GPR174 及其趋化因子配体 CCL21。我们已经揭示了一种机制,通过该机制可以微调 B 细胞生理学,从而将性二态性赋予体液免疫。男性和女性 B 细胞显示出不同的定位和促进生发中心的能力,其方式取决于 G 蛋白偶联引导受体 GPR174 及其趋化因子配体 CCL21。我们已经揭示了一种机制,通过该机制可以微调 B 细胞生理学,从而将性二态性赋予体液免疫。男性和女性 B 细胞显示出不同的定位和促进生发中心的能力,其方式取决于 G 蛋白偶联引导受体 GPR174 及其趋化因子配体 CCL21。
更新日期:2019-12-25
中文翻译:

GPR174-CCL21 模块赋予体液免疫性二态性
男性对免疫和感染的体液免疫反应以及对抗体介导的自身免疫的易感性通常较低 1 – 3 。然而,这种性别二态性的潜在机制尚不清楚。在这里,我们表明在雄性和雌性小鼠中产生生发中心的 B 细胞之间存在内在差异。我们发现抗原激活的雄性 B 细胞不能像雌性 B 细胞那样有效地将自身定位在次级淋巴器官的卵泡中心,在这些器官中,生发中心通常会发育。此外,GPR174——一种 X 染色体编码的 G 蛋白偶联受体——抑制雄性小鼠而不是雌性小鼠的生发中心的形成。这种效应是 B 细胞固有的,并且与 GPR174 增强的 B 细胞定位于毛囊的 T 细胞-B 细胞边界相关,以及来自 S1PR2 驱动的卵泡中心定位的男性而非女性 B 细胞的注意力分散。以 GPR174 依赖性方式诱导 B 细胞迁移的条件培养基的生化分级将 CCL21 鉴定为 GPR174 配体。响应 CCL21,GPR174 触发钙通量并优先诱导雄性 B 细胞的迁移;与女性 B 细胞相比,GPR174 还与男性中更多的 Gαi 蛋白相关。来自兰花切除小鼠的雄性 B 细胞表现出 GPR174 介导的 CCL21 迁移受损,而睾酮治疗可以挽救这种缺陷。来自睾酮处理小鼠的雌性 B 细胞表现出类似雄性的 GPR174-Gαi 关联和 GPR174 介导的迁移。从雄性 B 细胞中删除 GPR174 会更有效地定位到卵泡中心,更多生发中心的形成和对 B 细胞依赖性实验性自身免疫性脑脊髓炎的易感性增加。通过将 GPR174 鉴定为 CCL21 的受体并证明其对 B 细胞定位和参与生发中心的性别依赖性控制,我们揭示了一种机制,通过该机制可以微调 B 细胞生理学以赋予体液免疫性二态性。男性和女性 B 细胞显示出不同的定位和促进生发中心的能力,其方式取决于 G 蛋白偶联引导受体 GPR174 及其趋化因子配体 CCL21。我们已经揭示了一种机制,通过该机制可以微调 B 细胞生理学,从而将性二态性赋予体液免疫。男性和女性 B 细胞显示出不同的定位和促进生发中心的能力,其方式取决于 G 蛋白偶联引导受体 GPR174 及其趋化因子配体 CCL21。我们已经揭示了一种机制,通过该机制可以微调 B 细胞生理学,从而将性二态性赋予体液免疫。男性和女性 B 细胞显示出不同的定位和促进生发中心的能力,其方式取决于 G 蛋白偶联引导受体 GPR174 及其趋化因子配体 CCL21。



























 京公网安备 11010802027423号
京公网安备 11010802027423号