当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile synthesis of 2-alkynyl oxazoles via a Ce(OTf)3-catalyzed cascade reaction of alkynyl carboxylic acids with tert-butyl isocyanide.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9ob02337b Ming Cao 1 , Qing-Hu Teng 2 , Zhi-Wei Xi 1 , Li-Qiu Liu 1 , Ren-Yong Gu 1 , Ying-Chun Wang 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9ob02337b Ming Cao 1 , Qing-Hu Teng 2 , Zhi-Wei Xi 1 , Li-Qiu Liu 1 , Ren-Yong Gu 1 , Ying-Chun Wang 1
Affiliation
|
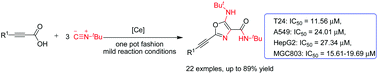
We developed an efficient and novel protocol to synthesize 2-alkynyl oxazoles from tert-butyl isocyanide and alkynyl carboxylic acids. This method allowed the synthesis of diversely functionalized oxazoles under mild reaction conditions, coupled with operational simplicity and these functionalized oxazoles showed a certain degree of biological activity. Moreover, compounds 2b, 2h, 2k, 2n, 2p and 2t exhibited good anticancer activities in human gastric cancer cells (MGC803) and human bladder tumor cells (T24), with IC50 below 20.0 μM.
中文翻译:

通过Ce(OTf)3催化的炔基羧酸与叔丁基异氰酸酯的级联反应,可轻松合成2-炔基恶唑。
我们开发了一种有效且新颖的方案,可从叔丁基异氰化物和炔基羧酸合成2-炔基恶唑。该方法允许在温和的反应条件下合成各种功能化的恶唑,并且操作简便,并且这些功能化的恶唑显示出一定程度的生物活性。此外,化合物2b,2h,2k,2n,2p和2t在人胃癌细胞(MGC803)和人膀胱肿瘤细胞(T24)中表现出良好的抗癌活性,IC50低于20.0μM。
更新日期:2020-02-10
中文翻译:

通过Ce(OTf)3催化的炔基羧酸与叔丁基异氰酸酯的级联反应,可轻松合成2-炔基恶唑。
我们开发了一种有效且新颖的方案,可从叔丁基异氰化物和炔基羧酸合成2-炔基恶唑。该方法允许在温和的反应条件下合成各种功能化的恶唑,并且操作简便,并且这些功能化的恶唑显示出一定程度的生物活性。此外,化合物2b,2h,2k,2n,2p和2t在人胃癌细胞(MGC803)和人膀胱肿瘤细胞(T24)中表现出良好的抗癌活性,IC50低于20.0μM。


























 京公网安备 11010802027423号
京公网安备 11010802027423号