当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Staphylococcus aureus IsdH Receptor Forms a Dynamic Complex with Human Hemoglobin that Triggers Heme Release via Two Distinct Hot Spots.
Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.jmb.2019.12.023 Ken Ellis-Guardiola 1 , Joseph Clayton 2 , Clarissa Pham 1 , Brendan J Mahoney 1 , Jeff Wereszczynski 2 , Robert T Clubb 3
Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.jmb.2019.12.023 Ken Ellis-Guardiola 1 , Joseph Clayton 2 , Clarissa Pham 1 , Brendan J Mahoney 1 , Jeff Wereszczynski 2 , Robert T Clubb 3
Affiliation
|
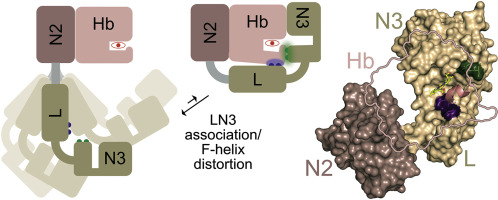
Iron is an essential nutrient that is actively acquired by bacterial pathogens during infections. Clinically important Staphylococcus aureus obtains iron by extracting heme from hemoglobin (Hb) using the closely related IsdB and IsdH surface receptors. In IsdH, extraction is mediated by a conserved tridomain unit that contains its second (N2) and third (N3) NEAT domains joined by a helical linker, called IsdHN2N3. Leveraging the crystal structure of the IsdHN2N3:Hb complex, we have probed the mechanism of heme capture using NMR, stopped-flow transfer kinetics measurements, and molecular dynamics (MD) simulations. NMR studies of the 220 kDa IsdHN2N3:Hb complex reveal that it is dynamic, with persistent interdomain motions enabling the linker and N3 domains in the receptor to transiently engage Hb to remove its heme. An alanine mutagenesis analysis reveals that two receptor subsites positioned ~20 Å apart trigger heme release by contacting Hb's F-helix. These subsites are located within the N3 and linker domains and appear to play distinct roles in stabilizing the heme transfer transition state. Linker domain contacts primarily function to destabilize Hb-heme interactions, thereby lowering ΔH‡, while contacts from the N3 subsite play a similar destabilizing role, but also form a bridge through which heme moves from Hb to the receptor. Interestingly, MD simulations suggest that within the transiently forming interface, both the F-helix and receptor bridge are in motion, dynamically sampling conformations that are suitable for heme transfer. Thus, IsdH triggers heme release from Hb via a flexible, low-affinity interface that forms fleetingly in solution.
中文翻译:

金黄色葡萄球菌IsdH受体与人类血红蛋白形成动态复合物,该复合物通过两个不同的热点触发血红素的释放。
铁是一种必需营养素,在感染过程中会被细菌病原体积极吸收。临床上重要的金黄色葡萄球菌通过使用密切相关的IsdB和IsdH表面受体从血红蛋白(Hb)中提取血红素来获得铁。在IsdH中,提取是由一个保守的三域单元介导的,该单元包含其第二个(N2)和第三个(N3)NEAT域,该域由一个称为IsdHN2N3的螺旋接头连接。利用IsdHN2N3:Hb络合物的晶体结构,我们使用NMR,停止流转移动力学测量和分子动力学(MD)模拟探索了血红素捕获的机理。对220 kDa IsdHN2N3:Hb络合物的NMR研究表明,它是动态的,具有持久的域间运动,使受体中的接头和N3域能够瞬时接合Hb以除去其血红素。丙氨酸诱变分析显示,通过与Hb的F螺旋接触,相距约20Å的两个受体亚位点触发了血红素的释放。这些子位点位于N3和接头域内,并在稳定血红素转移过渡状态中起着不同的作用。接头域接触主要起稳定Hb-血红素相互作用的作用,从而降低ΔH‡,而来自N3亚位点的接触起类似的稳定作用,但也形成血红素从Hb迁移至受体的桥。有趣的是,MD模拟表明,在短暂形成的界面内,F螺旋和受体桥均处于运动状态,可以动态采样适合血红素转移的构象。因此,IsdH通过灵活的低亲和力接口触发血红素从血红蛋白释放,该接口在解决方案中迅速形成。
更新日期:2019-12-25
中文翻译:

金黄色葡萄球菌IsdH受体与人类血红蛋白形成动态复合物,该复合物通过两个不同的热点触发血红素的释放。
铁是一种必需营养素,在感染过程中会被细菌病原体积极吸收。临床上重要的金黄色葡萄球菌通过使用密切相关的IsdB和IsdH表面受体从血红蛋白(Hb)中提取血红素来获得铁。在IsdH中,提取是由一个保守的三域单元介导的,该单元包含其第二个(N2)和第三个(N3)NEAT域,该域由一个称为IsdHN2N3的螺旋接头连接。利用IsdHN2N3:Hb络合物的晶体结构,我们使用NMR,停止流转移动力学测量和分子动力学(MD)模拟探索了血红素捕获的机理。对220 kDa IsdHN2N3:Hb络合物的NMR研究表明,它是动态的,具有持久的域间运动,使受体中的接头和N3域能够瞬时接合Hb以除去其血红素。丙氨酸诱变分析显示,通过与Hb的F螺旋接触,相距约20Å的两个受体亚位点触发了血红素的释放。这些子位点位于N3和接头域内,并在稳定血红素转移过渡状态中起着不同的作用。接头域接触主要起稳定Hb-血红素相互作用的作用,从而降低ΔH‡,而来自N3亚位点的接触起类似的稳定作用,但也形成血红素从Hb迁移至受体的桥。有趣的是,MD模拟表明,在短暂形成的界面内,F螺旋和受体桥均处于运动状态,可以动态采样适合血红素转移的构象。因此,IsdH通过灵活的低亲和力接口触发血红素从血红蛋白释放,该接口在解决方案中迅速形成。

























 京公网安备 11010802027423号
京公网安备 11010802027423号