当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, structure, and electrochemical performances of a novel three-dimensional framework K2[Cu(SO4)2]
Solid State Sciences ( IF 3.5 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.solidstatesciences.2019.106104 H. Andrew Zhou , Zhen Liu , Simon S. Ang , Jian-Jun Zhang
Solid State Sciences ( IF 3.5 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.solidstatesciences.2019.106104 H. Andrew Zhou , Zhen Liu , Simon S. Ang , Jian-Jun Zhang
|
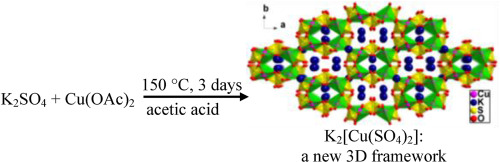
Abstract Among Cu2+-based electrode materials, copper sulfates have demonstrated significantly diverse electrochemical performances upon reactions with Li+ and Na+. Here we report a new, pure 3D framework K2[Cu(SO4)2] (1) bearing channels of both 12- and 8-membered rings and a new, facile synthesis of a Natrochalcite-type, 2D framework K[Cu2(OH)(H2O)(SO4)2] (2). To our best knowledge, the solvothermal synthesis of 1 represents the first example of making a pure-phase of such bimetallic copper sulfates containing no H2O and/or OH− groups via solution routes. This synthetic method can be generalized to develop more bimetallic sulfates of transition metal ions. Their electrochemical performances upon reactions with Na+ were preliminarily investigated and compared to that of Krohnkite, 1D framework Na2[Cu(SO4)2(H2O)2] (3). 1–3 decompose in the first discharge below 0.70 V (vs. Na+/Na) to deliver 62, 97, 67 mAh/g and continue to decompose in subsequent cycling, leading to low stable capacities of 12, 16, and 13 mAh/g respectively. Here we initiate the use of the HSAB, i.e., hard and soft (Lewis) acid and base, principle to explain the cycling stability difference among Cu-based frameworks and the difference between Cu-based and non-Cu-based frameworks in general in the hope to find directions to the development of Cu-based electrode materials.
中文翻译:

新型三维骨架K2[Cu(SO4)2]的合成、结构和电化学性能
摘要 在 Cu2+ 基电极材料中,硫酸铜在与 Li+ 和 Na+ 反应时表现出显着不同的电化学性能。在这里,我们报告了一种新的纯 3D 骨架 K2[Cu(SO4)2] (1) 轴承通道,包括 12 元环和 8 元环,以及一种新的、简便的钠钛矿型 2D 骨架 K[Cu2(OH) )(H2O)(SO4)2] (2)。据我们所知,1 的溶剂热合成代表了通过溶液途径制备不含 H2O 和/或 OH− 基团的这种双金属硫酸铜纯相的第一个例子。这种合成方法可以推广到开发更多的过渡金属离子的双金属硫酸盐。初步研究了它们与 Na+ 反应时的电化学性能,并与一维骨架 Na2[Cu(SO4)2(H2O)2] (3) 的 Krohnkite 的电化学性能进行了比较。1-3 在低于 0.70 V(vs. Na+/Na)的第一次放电中分解以提供 62、97、67 mAh/g 并在随后的循环中继续分解,导致 12、16 和 13 mAh/的低稳定容量g 分别。在这里,我们开始使用HSAB,即硬和软(路易斯)酸和碱,原理来解释Cu基框架之间的循环稳定性差异以及Cu基和非Cu基框架之间的一般差异希望为铜基电极材料的发展找到方向。
更新日期:2020-02-01
中文翻译:

新型三维骨架K2[Cu(SO4)2]的合成、结构和电化学性能
摘要 在 Cu2+ 基电极材料中,硫酸铜在与 Li+ 和 Na+ 反应时表现出显着不同的电化学性能。在这里,我们报告了一种新的纯 3D 骨架 K2[Cu(SO4)2] (1) 轴承通道,包括 12 元环和 8 元环,以及一种新的、简便的钠钛矿型 2D 骨架 K[Cu2(OH) )(H2O)(SO4)2] (2)。据我们所知,1 的溶剂热合成代表了通过溶液途径制备不含 H2O 和/或 OH− 基团的这种双金属硫酸铜纯相的第一个例子。这种合成方法可以推广到开发更多的过渡金属离子的双金属硫酸盐。初步研究了它们与 Na+ 反应时的电化学性能,并与一维骨架 Na2[Cu(SO4)2(H2O)2] (3) 的 Krohnkite 的电化学性能进行了比较。1-3 在低于 0.70 V(vs. Na+/Na)的第一次放电中分解以提供 62、97、67 mAh/g 并在随后的循环中继续分解,导致 12、16 和 13 mAh/的低稳定容量g 分别。在这里,我们开始使用HSAB,即硬和软(路易斯)酸和碱,原理来解释Cu基框架之间的循环稳定性差异以及Cu基和非Cu基框架之间的一般差异希望为铜基电极材料的发展找到方向。


























 京公网安备 11010802027423号
京公网安备 11010802027423号