当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organocatalytic enantioselective synthesis of 2,5,5-trisubstituted piperidines bearing a quaternary stereocenter. Vinyl sulfonamide as a new amine protecting group.
Chemical Communications ( IF 4.9 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9cc09113k Marcos Escolano 1 , Marta Guerola 1 , Javier Torres 1 , Daniel Gaviña 1 , Gloria Alzuet-Piña 2 , María Sánchez-Rosello 1 , Carlos Del Pozo 1
Chemical Communications ( IF 4.9 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9cc09113k Marcos Escolano 1 , Marta Guerola 1 , Javier Torres 1 , Daniel Gaviña 1 , Gloria Alzuet-Piña 2 , María Sánchez-Rosello 1 , Carlos Del Pozo 1
Affiliation
|
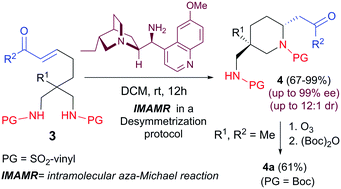
An organocatalytic desymmetrizing intramolecular aza-Michael reaction with vinyl sulfonamides as nucleophilic nitrogen source has been devised for the synthesis of a new family of 2,5,5-trisubstituted piperidines bearing a quaternary sterocenter. The process takes place with excellent levels of enantioselectivity and moderate to good diastereoselectivity. The vinyl sulfonamide moiety can be removed by means of an ozonolysis reaction.
中文翻译:

带有季立体中心的2,5,5-三取代的哌啶的有机催化对映选择性合成。乙烯基磺酰胺作为新的胺保护基。
已经设计了一种以乙烯基磺酰胺作为亲核氮源的有机催化去对称化分子内氮杂-迈克尔反应,用于合成带有季体中心的新的2,5,5-三取代哌啶家族。该过程以优异的对映选择性和中等至良好的非对映选择性进行。可以通过臭氧分解反应除去乙烯基磺酰胺部分。
更新日期:2020-01-08
中文翻译:

带有季立体中心的2,5,5-三取代的哌啶的有机催化对映选择性合成。乙烯基磺酰胺作为新的胺保护基。
已经设计了一种以乙烯基磺酰胺作为亲核氮源的有机催化去对称化分子内氮杂-迈克尔反应,用于合成带有季体中心的新的2,5,5-三取代哌啶家族。该过程以优异的对映选择性和中等至良好的非对映选择性进行。可以通过臭氧分解反应除去乙烯基磺酰胺部分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号