当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
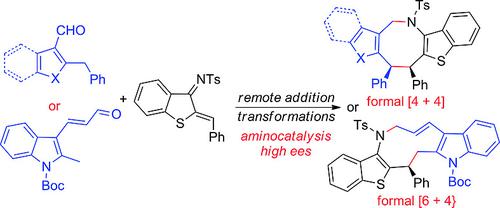
Asymmetric Remote Addition Reactions of Heterocycle‐Based Dearomative Dienamine or Trienamine Species to 1‐Azadienes: Application to Construct Chiral Azocanes and Azecanes
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-01-21 , DOI: 10.1002/ejoc.201901848 Di Hu 1 , Yang Gao 1 , Xue Song 1 , Wei Du 1 , Ying-Chun Chen 1, 2
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-01-21 , DOI: 10.1002/ejoc.201901848 Di Hu 1 , Yang Gao 1 , Xue Song 1 , Wei Du 1 , Ying-Chun Chen 1, 2
Affiliation
|
Medium‐sized heterocycles: A few dearomative dienamine or even trienamine species based on heteroaryl aldehydes underwent highly regio‐, chemo‐, and stereoselective remote Michael additions to 1‐azadienes. The resulting multifunctional adducts enabled the efficient construction of enantioenriched chiral azocane or even azecane frameworks fused with diverse heterocycles.

中文翻译:

杂环基脱芳香二烯胺或三烯胺物种与1-Azadienes的不对称远程加成反应:在构建手性氮杂环丁烷和氮杂环戊烷中的应用
中型杂环:少数基于杂芳基醛的脱芳香二烯胺或三烯胺种类对1-氮杂二烯进行了高度区域,化学和立体选择性的远程Michael加成。所得的多功能加合物能够有效构建富含对映体的手性偶氮烷,甚至与各种杂环稠合的氮杂环庚烷骨架。

更新日期:2020-01-22

中文翻译:

杂环基脱芳香二烯胺或三烯胺物种与1-Azadienes的不对称远程加成反应:在构建手性氮杂环丁烷和氮杂环戊烷中的应用
中型杂环:少数基于杂芳基醛的脱芳香二烯胺或三烯胺种类对1-氮杂二烯进行了高度区域,化学和立体选择性的远程Michael加成。所得的多功能加合物能够有效构建富含对映体的手性偶氮烷,甚至与各种杂环稠合的氮杂环庚烷骨架。



























 京公网安备 11010802027423号
京公网安备 11010802027423号