当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bacterial lipase triggers the release of antibiotics from digestible liquid crystal nanoparticles.
Journal of Controlled Release ( IF 10.8 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.jconrel.2019.12.037 Chelsea R Thorn 1 , Andrew J Clulow 2 , Ben J Boyd 3 , Clive A Prestidge 4 , Nicky Thomas 1
Journal of Controlled Release ( IF 10.8 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.jconrel.2019.12.037 Chelsea R Thorn 1 , Andrew J Clulow 2 , Ben J Boyd 3 , Clive A Prestidge 4 , Nicky Thomas 1
Affiliation
|
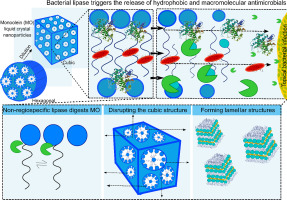
In the advent of the post-antibiotic era, new strategies are urgently required to improve the efficacy of antimicrobials and outsmart multi-drug resistant bacteria. Exploiting a basic survival mechanism of bacteria, lipase production, monoolein liquid crystal nanoparticles (MO-LCNPs) were investigated as a bacterial-triggered drug delivery system for three different antimicrobial compounds and compared with model sn-1/3 regiospecific and non-regiospecific lipases via pH-stat titration, proton nuclear magnetic resonance and in situ synchrotron small-angle X-ray scattering. The release of model hydrophobic (rifampicin) and macromolecular (alginate lyase) antimicrobials were triggered from MO-LCNPs at 82-fold and 7-fold higher rates (respectively) due to bacterial lipase digestion of MO-LCNPs, which could not be stimulated with a small hydrophilic antibiotic (ciprofloxacin HCl) or non-digestible, phytantriol-LCNPs. While sn-1/3 regiospecific lipase rapidly digested MO-LCNPs in a two-phase process, the single-phase digestion kinetics of the non-regiospecific lipase steadily digested the cubic Im3m structure and gave rise to lamellar structures that ultimately stimulated the triggered antibiotic release. Accordingly, MO-LCNPs have an application for localised Pseudomonas aeruginosa and Staphylococcus aureus infections that produce non-regiospecific lipases and for concentration-dependent antibiotics that have macromolecular (MW ~ 30 kDa) or hydrophobic (logP ~ 4) chemistries, as a triggered bolus release would be clinically efficacious for improved bacterial eradication.
中文翻译:

细菌脂肪酶触发了可消化液晶纳米颗粒中抗生素的释放。
在后抗生素时代的到来中,迫切需要新的策略来提高抗菌剂和优于多药耐药菌的功效。利用细菌的基本生存机制,脂肪酶的产生,单油精液晶纳米颗粒(MO-LCNPs)作为细菌触发的三种不同抗菌化合物的药物传递系统进行了研究,并与模型sn-1 / 3区域特异性和非区域特异性脂肪酶进行了比较通过pH静态滴定,质子核磁共振和原位同步加速器小角X射线散射。由于MO-LCNP的细菌脂肪酶消化作用,MO-LCNP分别触发了模型疏水性(利福平)和大分子(海藻酸裂解酶)抗微生物剂的释放,分别释放了82倍和7倍。可用小型亲水性抗生素(盐酸环丙沙星)或不可消化的植物三醇-LCNP刺激。当sn-1 / 3区域特异性脂肪酶在两阶段过程中快速消化MO-LCNPs时,非区域特异性脂肪酶的单相消化动力学稳定地消化了立方Im3m结构,并产生了层状结构,最终刺激了触发的抗生素释放。因此,MO-LCNPs可用于产生非区域特异性脂肪酶的铜绿假单胞菌和金黄色葡萄球菌感染,以及用于大剂量(MW〜30 kDa)或疏水性(logP〜4)化学物质的浓度依赖性抗生素作为触发药丸释放对于改善细菌的根除在临床上将是有效的。当sn-1 / 3区域特异性脂肪酶在两阶段过程中快速消化MO-LCNPs时,非区域特异性脂肪酶的单相消化动力学稳定地消化了立方Im3m结构并产生了层状结构,最终刺激了触发的抗生素释放。因此,MO-LCNPs可用于产生非区域特异性脂肪酶的铜绿假单胞菌和金黄色葡萄球菌感染,以及用于大剂量(MW〜30 kDa)或疏水性(logP〜4)化学物质的浓度依赖性抗生素作为触发药丸释放对于改善细菌的根除在临床上将是有效的。当sn-1 / 3区域特异性脂肪酶在两阶段过程中快速消化MO-LCNPs时,非区域特异性脂肪酶的单相消化动力学稳定地消化了立方Im3m结构并产生了层状结构,最终刺激了触发的抗生素释放。因此,MO-LCNPs可用于产生非区域特异性脂肪酶的铜绿假单胞菌和金黄色葡萄球菌感染,以及用于大剂量(MW〜30 kDa)或疏水性(logP〜4)化学物质的浓度依赖性抗生素作为触发药丸释放对于改善细菌的根除在临床上将是有效的。非区域特异性脂肪酶的单相消化动力学稳定地消化了立方的Im3m结构,并形成了层状结构,最终刺激了触发的抗生素释放。因此,MO-LCNPs可用于产生非区域特异性脂肪酶的铜绿假单胞菌和金黄色葡萄球菌感染,以及用于大剂量(MW〜30 kDa)或疏水性(logP〜4)化学物质的浓度依赖性抗生素作为触发药丸释放对于改善细菌的根除在临床上将是有效的。非区域特异性脂肪酶的单相消化动力学稳定地消化了立方的Im3m结构,并形成了层状结构,最终刺激了触发的抗生素释放。因此,MO-LCNPs可用于产生非区域特异性脂肪酶的铜绿假单胞菌和金黄色葡萄球菌感染,以及用于大剂量(MW〜30 kDa)或疏水性(logP〜4)化学物质的浓度依赖性抗生素作为触发药丸释放对于改善细菌的根除在临床上将是有效的。
更新日期:2019-12-25
中文翻译:

细菌脂肪酶触发了可消化液晶纳米颗粒中抗生素的释放。
在后抗生素时代的到来中,迫切需要新的策略来提高抗菌剂和优于多药耐药菌的功效。利用细菌的基本生存机制,脂肪酶的产生,单油精液晶纳米颗粒(MO-LCNPs)作为细菌触发的三种不同抗菌化合物的药物传递系统进行了研究,并与模型sn-1 / 3区域特异性和非区域特异性脂肪酶进行了比较通过pH静态滴定,质子核磁共振和原位同步加速器小角X射线散射。由于MO-LCNP的细菌脂肪酶消化作用,MO-LCNP分别触发了模型疏水性(利福平)和大分子(海藻酸裂解酶)抗微生物剂的释放,分别释放了82倍和7倍。可用小型亲水性抗生素(盐酸环丙沙星)或不可消化的植物三醇-LCNP刺激。当sn-1 / 3区域特异性脂肪酶在两阶段过程中快速消化MO-LCNPs时,非区域特异性脂肪酶的单相消化动力学稳定地消化了立方Im3m结构,并产生了层状结构,最终刺激了触发的抗生素释放。因此,MO-LCNPs可用于产生非区域特异性脂肪酶的铜绿假单胞菌和金黄色葡萄球菌感染,以及用于大剂量(MW〜30 kDa)或疏水性(logP〜4)化学物质的浓度依赖性抗生素作为触发药丸释放对于改善细菌的根除在临床上将是有效的。当sn-1 / 3区域特异性脂肪酶在两阶段过程中快速消化MO-LCNPs时,非区域特异性脂肪酶的单相消化动力学稳定地消化了立方Im3m结构并产生了层状结构,最终刺激了触发的抗生素释放。因此,MO-LCNPs可用于产生非区域特异性脂肪酶的铜绿假单胞菌和金黄色葡萄球菌感染,以及用于大剂量(MW〜30 kDa)或疏水性(logP〜4)化学物质的浓度依赖性抗生素作为触发药丸释放对于改善细菌的根除在临床上将是有效的。当sn-1 / 3区域特异性脂肪酶在两阶段过程中快速消化MO-LCNPs时,非区域特异性脂肪酶的单相消化动力学稳定地消化了立方Im3m结构并产生了层状结构,最终刺激了触发的抗生素释放。因此,MO-LCNPs可用于产生非区域特异性脂肪酶的铜绿假单胞菌和金黄色葡萄球菌感染,以及用于大剂量(MW〜30 kDa)或疏水性(logP〜4)化学物质的浓度依赖性抗生素作为触发药丸释放对于改善细菌的根除在临床上将是有效的。非区域特异性脂肪酶的单相消化动力学稳定地消化了立方的Im3m结构,并形成了层状结构,最终刺激了触发的抗生素释放。因此,MO-LCNPs可用于产生非区域特异性脂肪酶的铜绿假单胞菌和金黄色葡萄球菌感染,以及用于大剂量(MW〜30 kDa)或疏水性(logP〜4)化学物质的浓度依赖性抗生素作为触发药丸释放对于改善细菌的根除在临床上将是有效的。非区域特异性脂肪酶的单相消化动力学稳定地消化了立方的Im3m结构,并形成了层状结构,最终刺激了触发的抗生素释放。因此,MO-LCNPs可用于产生非区域特异性脂肪酶的铜绿假单胞菌和金黄色葡萄球菌感染,以及用于大剂量(MW〜30 kDa)或疏水性(logP〜4)化学物质的浓度依赖性抗生素作为触发药丸释放对于改善细菌的根除在临床上将是有效的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号