Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Association of body mass index and cardiotoxicity related to anthracyclines and trastuzumab in early breast cancer: French CANTO cohort study.
PLOS Medicine ( IF 15.8 ) Pub Date : 2019-12-23 , DOI: 10.1371/journal.pmed.1002989 Elisé G Kaboré 1 , Charles Guenancia 2 , Ines Vaz-Luis 3 , Antonio Di Meglio 3 , Barbara Pistilli 3 , Charles Coutant 4 , Paul Cottu 5 , Anne Lesur 6 , Thierry Petit 7 , Florence Dalenc 8 , Philippe Rouanet 9 , Antoine Arnaud 10 , Olivier Arsene 11 , Mahmoud Ibrahim 12 , Johanna Wassermann 13 , Geneviève Boileau-Jolimoy 14 , Anne-Laure Martin 15 , Jérôme Lemonnier 15 , Fabrice André 3 , Patrick Arveux 1, 4
PLOS Medicine ( IF 15.8 ) Pub Date : 2019-12-23 , DOI: 10.1371/journal.pmed.1002989 Elisé G Kaboré 1 , Charles Guenancia 2 , Ines Vaz-Luis 3 , Antonio Di Meglio 3 , Barbara Pistilli 3 , Charles Coutant 4 , Paul Cottu 5 , Anne Lesur 6 , Thierry Petit 7 , Florence Dalenc 8 , Philippe Rouanet 9 , Antoine Arnaud 10 , Olivier Arsene 11 , Mahmoud Ibrahim 12 , Johanna Wassermann 13 , Geneviève Boileau-Jolimoy 14 , Anne-Laure Martin 15 , Jérôme Lemonnier 15 , Fabrice André 3 , Patrick Arveux 1, 4
Affiliation
|
BACKGROUND
In patients treated with cardiotoxic chemotherapies, the presence of cardiovascular risk factors and previous cardiac disease have been strongly correlated to the onset of cardiotoxicity. The influence of overweight and obesity as risk factors in the development of treatment-related cardiotoxicity in breast cancer (BC) was recently suggested. However, due to meta-analysis design, it was not possible to take into account associated cardiac risk factors or other classic risk factors for anthracycline (antineoplastic antibiotic) and trastuzumab (monoclonal antibody) cardiotoxicity.
METHODS AND FINDINGS
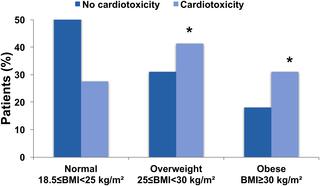
Using prospective data collected from 2012-2014 in the French national multicenter prospective CANTO (CANcer TOxicities) study of 26 French cancer centers, we aimed to examine the association of body mass index (BMI) and cardiotoxicity (defined as a reduction in left ventricular ejection fraction [LVEF] > 10 percentage points from baseline to LVEF < 50%). In total, 929 patients with stage I-III BC (mean age 52 ± 11 years, mean BMI 25.6 ± 5.1 kg/m2, 42% with 1 or more cardiovascular risk factors) treated with anthracycline (86% epirubicin, 7% doxorubicin) and/or trastuzumab (36%), with LVEF measurement at baseline and at least 1 assessment post-chemotherapy were eligible in this interim analysis. We analyzed associations between BMI and cardiotoxicity using multivariate logistic regression. At baseline, nearly 50% of the study population was overweight or obese. During a mean follow-up of 22 ± 2 months following treatment completion, cardiotoxicity occurred in 29 patients (3.2%). The obese group was more prone to cardiotoxicity than the normal-weight group (9/171 versus 8/466; p = 0.01). In multivariate analysis, obesity (odds ratio [OR] 3.02; 95% CI 1.10-8.25; p = 0.03) and administration of trastuzumab (OR 12.12; 95% CI 3.6-40.4; p < 0.001) were independently associated with cardiotoxicity. Selection bias and relatively short follow-up are potential limitations of this national multicenter observational cohort.
CONCLUSIONS
In BC patients, obesity appears to be associated with an important increase in risk-related cardiotoxicity (CANTO, ClinicalTrials.gov registry ID: NCT01993498).
TRIAL REGISTRATION
ClinicalTrials.gov NCT01993498.
中文翻译:

早期乳腺癌中与蒽环类药物和曲妥珠单抗相关的体重指数与心脏毒性的关联:法国CANTO队列研究。
背景技术在接受心脏毒性化学疗法治疗的患者中,心血管危险因素的存在和先前的心脏病已与心脏毒性的发作密切相关。最近有人建议将超重和肥胖作为危险因素影响乳腺癌(BC)与治疗有关的心脏毒性的发展。但是,由于进行荟萃分析设计,因此无法考虑相关的心脏危险因素或其他蒽环类抗生素(抗肿瘤抗生素)和曲妥珠单抗(单克隆抗体)心脏毒性的典型危险因素。方法和研究结果使用2012年至2014年在法国国家多中心26个法国癌症中心的前瞻性CANTO(癌症毒性)研究中收集的前瞻性数据,我们的目的是检查体重指数(BMI)与心脏毒性的关系(定义为左室射血分数[LVEF]从基线降低到LVEF <50%> 10个百分点)。共有929名接受蒽环类抗生素治疗的I-III期BC患者(平均年龄52±11岁,平均BMI 25.6±5.1 kg / m2,42%患有1种或多种心血管危险因素)(86%表柔比星,7%阿霉素)和/或曲妥珠单抗(36%),基线时进行LVEF测定,化疗后至少进行1次评估符合该中期分析的条件。我们使用多元逻辑回归分析了BMI与心脏毒性之间的关联。在基线时,将近50%的研究人群超重或肥胖。在完成治疗后22±2个月的平均随访中,有29名患者发生了心脏毒性(3.2%)。肥胖组比正常体重组更容易发生心脏毒性(9/171对8/466; p = 0.01)。在多变量分析中,肥胖(赔率[OR] 3.02; 95%CI 1.10-8.25; p = 0.03)和曲妥珠单抗的给药(OR 12.12; 95%CI 3.6-40.4; p <0.001)与心脏毒性独立相关。选择偏倚和相对较短的随访是该国家多中心观察队列的潜在局限性。结论在BC患者中,肥胖似乎与风险相关的心脏毒性的重要增加有关(CANTO,ClinicalTrials.gov注册ID:NCT01993498)。试验注册ClinicalTrials.gov NCT01993498。03)和曲妥珠单抗的给药(OR 12.12; 95%CI 3.6-40.4; p <0.001)与心脏毒性独立相关。选择偏倚和相对较短的随访是该国家多中心观察队列的潜在局限性。结论在BC患者中,肥胖似乎与风险相关的心脏毒性的重要增加有关(CANTO,ClinicalTrials.gov注册ID:NCT01993498)。试验注册ClinicalTrials.gov NCT01993498。03)和曲妥珠单抗的给药(OR 12.12; 95%CI 3.6-40.4; p <0.001)与心脏毒性独立相关。选择偏倚和相对较短的随访是该国家多中心观察队列的潜在局限性。结论在BC患者中,肥胖似乎与风险相关的心脏毒性的重要增加有关(CANTO,ClinicalTrials.gov注册ID:NCT01993498)。试验注册ClinicalTrials.gov NCT01993498。ClinicalTrials.gov注册表ID:NCT01993498)。试验注册ClinicalTrials.gov NCT01993498。ClinicalTrials.gov注册表ID:NCT01993498)。试验注册ClinicalTrials.gov NCT01993498。
更新日期:2020-01-14
中文翻译:

早期乳腺癌中与蒽环类药物和曲妥珠单抗相关的体重指数与心脏毒性的关联:法国CANTO队列研究。
背景技术在接受心脏毒性化学疗法治疗的患者中,心血管危险因素的存在和先前的心脏病已与心脏毒性的发作密切相关。最近有人建议将超重和肥胖作为危险因素影响乳腺癌(BC)与治疗有关的心脏毒性的发展。但是,由于进行荟萃分析设计,因此无法考虑相关的心脏危险因素或其他蒽环类抗生素(抗肿瘤抗生素)和曲妥珠单抗(单克隆抗体)心脏毒性的典型危险因素。方法和研究结果使用2012年至2014年在法国国家多中心26个法国癌症中心的前瞻性CANTO(癌症毒性)研究中收集的前瞻性数据,我们的目的是检查体重指数(BMI)与心脏毒性的关系(定义为左室射血分数[LVEF]从基线降低到LVEF <50%> 10个百分点)。共有929名接受蒽环类抗生素治疗的I-III期BC患者(平均年龄52±11岁,平均BMI 25.6±5.1 kg / m2,42%患有1种或多种心血管危险因素)(86%表柔比星,7%阿霉素)和/或曲妥珠单抗(36%),基线时进行LVEF测定,化疗后至少进行1次评估符合该中期分析的条件。我们使用多元逻辑回归分析了BMI与心脏毒性之间的关联。在基线时,将近50%的研究人群超重或肥胖。在完成治疗后22±2个月的平均随访中,有29名患者发生了心脏毒性(3.2%)。肥胖组比正常体重组更容易发生心脏毒性(9/171对8/466; p = 0.01)。在多变量分析中,肥胖(赔率[OR] 3.02; 95%CI 1.10-8.25; p = 0.03)和曲妥珠单抗的给药(OR 12.12; 95%CI 3.6-40.4; p <0.001)与心脏毒性独立相关。选择偏倚和相对较短的随访是该国家多中心观察队列的潜在局限性。结论在BC患者中,肥胖似乎与风险相关的心脏毒性的重要增加有关(CANTO,ClinicalTrials.gov注册ID:NCT01993498)。试验注册ClinicalTrials.gov NCT01993498。03)和曲妥珠单抗的给药(OR 12.12; 95%CI 3.6-40.4; p <0.001)与心脏毒性独立相关。选择偏倚和相对较短的随访是该国家多中心观察队列的潜在局限性。结论在BC患者中,肥胖似乎与风险相关的心脏毒性的重要增加有关(CANTO,ClinicalTrials.gov注册ID:NCT01993498)。试验注册ClinicalTrials.gov NCT01993498。03)和曲妥珠单抗的给药(OR 12.12; 95%CI 3.6-40.4; p <0.001)与心脏毒性独立相关。选择偏倚和相对较短的随访是该国家多中心观察队列的潜在局限性。结论在BC患者中,肥胖似乎与风险相关的心脏毒性的重要增加有关(CANTO,ClinicalTrials.gov注册ID:NCT01993498)。试验注册ClinicalTrials.gov NCT01993498。ClinicalTrials.gov注册表ID:NCT01993498)。试验注册ClinicalTrials.gov NCT01993498。ClinicalTrials.gov注册表ID:NCT01993498)。试验注册ClinicalTrials.gov NCT01993498。


























 京公网安备 11010802027423号
京公网安备 11010802027423号