当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Strengthening Peptoid Helicity through Sequence Site-Specific Positioning of Amide cis-Inducing NtBu Monomers.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.joc.9b02916 Maha Rzeigui 1, 2 , Mounir Traikia 1 , Laurent Jouffret 1 , Alexandre Kriznik 3, 4 , Jameleddine Khiari 2 , Olivier Roy 1 , Claude Taillefumier 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.joc.9b02916 Maha Rzeigui 1, 2 , Mounir Traikia 1 , Laurent Jouffret 1 , Alexandre Kriznik 3, 4 , Jameleddine Khiari 2 , Olivier Roy 1 , Claude Taillefumier 1
Affiliation
|
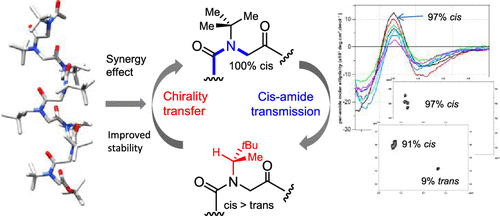
The synthesis of biomimetic helical secondary structures is sought after for the construction of innovative nanomaterials and applications in medicinal chemistry such as the development of protein-protein interaction modulators. Peptoids, a sequence-defined family of oligomers, enable a peptidomimetic strategy, especially considering the easily accessible monomer diversity and peptoid helical folding propensity. However, cis-trans isomerization of the backbone tertiary amides may impair the peptoid's adoption of stable secondary structures, notably the all-cis polyproline I-like helical conformation. Here, we show that cis-inducing NtBu achiral monomers strategically positioned within chiral sequences may reinforce the degree of peptoid helicity, although with a reduced content of chiral side chains. The design principles presented here will undoubtedly help achieve more conformationally stable helical peptoids with desired functions.
中文翻译:

通过酰胺顺式诱导NtBu单体的序列位点特异性定位来增强类肽螺旋度。
寻求仿生螺旋二级结构的合成,用于构建创新的纳米材料,并将其应用于药物化学,例如蛋白质-蛋白质相互作用调节剂的开发。类肽是序列定义的寡聚物家族,可实现拟肽策略,尤其是考虑到易于获得的单体多样性和类肽螺旋折叠倾向。但是,主链叔酰胺的顺反异构化可能会损害类肽对稳定二级结构的采用,尤其是全顺式脯氨酸I样螺旋构象。在这里,我们显示策略性地定位在手性序列内的顺式诱导NtBu非手性单体可能会增强类肽螺旋度,尽管手性侧链的含量降低。
更新日期:2020-01-13
中文翻译:

通过酰胺顺式诱导NtBu单体的序列位点特异性定位来增强类肽螺旋度。
寻求仿生螺旋二级结构的合成,用于构建创新的纳米材料,并将其应用于药物化学,例如蛋白质-蛋白质相互作用调节剂的开发。类肽是序列定义的寡聚物家族,可实现拟肽策略,尤其是考虑到易于获得的单体多样性和类肽螺旋折叠倾向。但是,主链叔酰胺的顺反异构化可能会损害类肽对稳定二级结构的采用,尤其是全顺式脯氨酸I样螺旋构象。在这里,我们显示策略性地定位在手性序列内的顺式诱导NtBu非手性单体可能会增强类肽螺旋度,尽管手性侧链的含量降低。


























 京公网安备 11010802027423号
京公网安备 11010802027423号