当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Analogs of A54145D and A54145A1 for Structure-Activity Relationship Studies.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.joc.9b02922 Braden Kralt 1 , Ryan Moreira 1 , Michael Palmer 1 , Scott D Taylor 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.joc.9b02922 Braden Kralt 1 , Ryan Moreira 1 , Michael Palmer 1 , Scott D Taylor 1
Affiliation
|
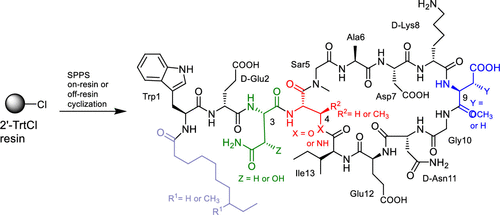
The total solid-phase synthesis and in vitro biological activity of a series of analogs of A54145 factor D (A5D) and A54145 factor A1 (A5A1), two cyclic lipodepsipeptide antibiotics, are reported. An on-resin cyclization strategy was employed to prepare A5A1 analogs in which Thr4, the residue involved in the depsi (ester) bond, was replaced with either diaminopropionic acid (DAPA), (2S,3R)-diaminobutyric acid (DABA), or serine, effectively replacing the ring-closing ester bond with an amide linkage or with a primary ester. Antibacterial studies with these four analogs revealed that, contrary to a previous report, replacing the ester bond with an amide bond significantly reduces biological activity, and that both the ester bond and the methyl group at the γ-position of Thr4 are crucial for activity. Consistent with literature reports, we found that the single substitution of either 3-hydroxyasparagine (HOAsn) or 3-methoxyaspartate (MeOAsp) with Asn or Asp, respectively, in A5D is more detrimental to activity than the double substitution where both HOAsn and MeOAsp are replaced with Asn or Asp, respectively.
中文翻译:

用于结构-活性关系研究的A54145D和A54145A1类似物的全合成。
据报道,两种环状脂肽肽抗生素A54145因子D(A5D)和A54145因子A1(A5A1)的一系列类似物的总固相合成和体外生物学活性。采用树脂上环化策略制备A5A1类似物,其中Thr4(参与depsi(酯)键的残基)被二氨基丙酸(DAPA),(2S,3R)-二氨基丁酸(DABA)或丝氨酸,有效地用酰胺键或伯酯取代了闭环酯键。用这四个类似物进行的抗菌研究表明,与先前的报道相反,用酰胺键取代酯键会大大降低生物活性,并且Thr4的γ位上的酯键和甲基对于活性至关重要。与文献报道一致,
更新日期:2020-01-13
中文翻译:

用于结构-活性关系研究的A54145D和A54145A1类似物的全合成。
据报道,两种环状脂肽肽抗生素A54145因子D(A5D)和A54145因子A1(A5A1)的一系列类似物的总固相合成和体外生物学活性。采用树脂上环化策略制备A5A1类似物,其中Thr4(参与depsi(酯)键的残基)被二氨基丙酸(DAPA),(2S,3R)-二氨基丁酸(DABA)或丝氨酸,有效地用酰胺键或伯酯取代了闭环酯键。用这四个类似物进行的抗菌研究表明,与先前的报道相反,用酰胺键取代酯键会大大降低生物活性,并且Thr4的γ位上的酯键和甲基对于活性至关重要。与文献报道一致,



























 京公网安备 11010802027423号
京公网安备 11010802027423号