当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reversible Reactions: Extent of Reaction and Theoretical Yield
Journal of Chemical Education ( IF 3 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.jchemed.9b00881 Igor Novak 1
Journal of Chemical Education ( IF 3 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.jchemed.9b00881 Igor Novak 1
Affiliation
|
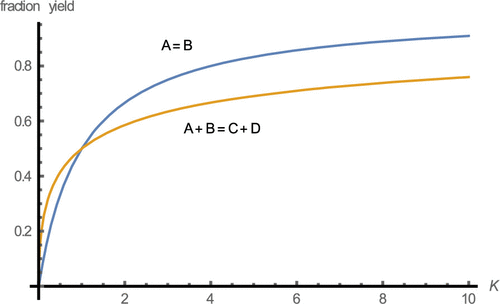
Theoretical yield and extent of reaction (degree of completion) are important concepts in stoichiometry and in chemical kinetics. We provide expressions describing theoretical yield (Y) and maximum extent of reaction (fractional yield, fmax) for five examples of commonly encountered reversible chemical reactions: A ⇌ B; A + B ⇌ C + D; A + B + C ⇌ D + E + F; A ⇌ B + C; and A + B ⇌ C. We discuss how the equilibrium constant and initial reactant concentration influence the maximum fractional yield (degree of reaction completion) and Y and illustrate their effects on specific examples of reversible reactions: conformer equilibria, esterification reaction, and ionization of weak acids.
中文翻译:

可逆反应:反应程度和理论产率
理论产率和反应程度(完成度)是化学计量和化学动力学中的重要概念。我们提供了五个表达式,用于描述五个常见的可逆化学反应实例的理论收率(Y)和最大反应程度(分数收率,f max):A + B⇌C + D; A + B + C⇌D + E + F; A⇌B+ C; 和A + B⇌C。我们讨论平衡常数和初始反应物浓度如何影响最大分数产率(反应完成度)和Y,并说明它们对可逆反应的具体实例的影响:构象平衡,酯化反应和离子的电离。弱酸。
更新日期:2019-12-25
中文翻译:

可逆反应:反应程度和理论产率
理论产率和反应程度(完成度)是化学计量和化学动力学中的重要概念。我们提供了五个表达式,用于描述五个常见的可逆化学反应实例的理论收率(Y)和最大反应程度(分数收率,f max):A + B⇌C + D; A + B + C⇌D + E + F; A⇌B+ C; 和A + B⇌C。我们讨论平衡常数和初始反应物浓度如何影响最大分数产率(反应完成度)和Y,并说明它们对可逆反应的具体实例的影响:构象平衡,酯化反应和离子的电离。弱酸。


























 京公网安备 11010802027423号
京公网安备 11010802027423号