当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kemp Elimination Reaction Catalyzed by Electric Fields.
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-01-17 , DOI: 10.1002/cphc.201901155 Carles Acosta-Silva 1 , Joan Bertran 1 , Vicenç Branchadell 1 , Antoni Oliva 1
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-01-17 , DOI: 10.1002/cphc.201901155 Carles Acosta-Silva 1 , Joan Bertran 1 , Vicenç Branchadell 1 , Antoni Oliva 1
Affiliation
|
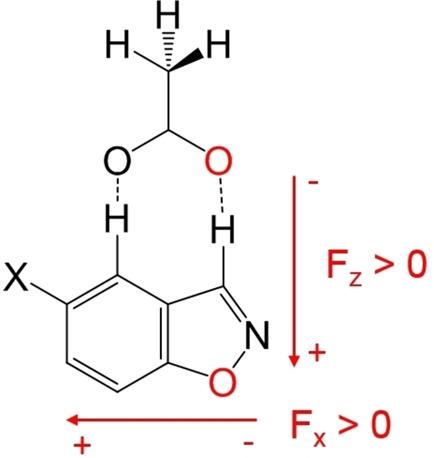
The Kemp elimination reaction is the most widely used in the de novo design of new enzymes. The effect of two different kinds of electric fields in the reactions of acetate as a base with benzisoxazole and 5‐nitrobenzisoxazole as substrates have been theoretically studied. The effect of the solvent reaction field has been calculated using the SMD continuum model for several solvents; we have shown that solvents inhibit both reactions, the decrease of the reaction rate being larger as far as the dielectric constant is increased. The diminution of the reaction rate is especially remarkable between aprotic organic solvents and protic solvents as water, the electrostatic term of the hydrogen bonds being the main factor for the large inhibitory effect of water. The presence of an external electric field oriented in the direction of the charge transfer (z axis) increases it and, so, the reaction rate. In the reaction of the nitro compound, if the electric field is oriented in an orthogonal direction (x axis) the charge transfer to the NO2 group is favored and there is a subsequent increase of the reaction rate. However, this increase is smaller than the one produced by the field in the z axis. It is worthwhile mentioning that one of the main effects of external electric fields of intermediate intensity is the reorientation of the reactants. Finally, the implications of our results in the de novo design of enzymes are discussed.
中文翻译:

电场催化的坎普消除反应。
肯普消除反应是新酶从头设计中使用最广泛的反应。从理论上研究了两种不同电场在乙酸根作为碱与苯并异恶唑和5-硝基苯并恶唑为底物的反应中的作用。已使用SMD连续模型对几种溶剂计算了溶剂反应场的影响。我们已经表明,溶剂抑制了两个反应,只要介电常数增加,反应速率的降低就更大。在非质子有机溶剂和质子溶剂如水之间,反应速率的降低尤为明显,氢键的静电项是水抑制作用大的主要因素。沿电荷转移方向(z轴)定向的外部电场的存在会增加电荷反应速率,从而提高反应速率。在硝基化合物的反应中,如果电场沿正交方向(x轴)取向,则电荷转移到NO2组是有利的,并且随后反应速率增加。但是,这种增加小于由z轴上的磁场产生的增加。值得一提的是,中等强度外部电场的主要作用之一是反应物的重新定向。最后,讨论了我们的结果在酶从头设计中的意义。
更新日期:2020-01-17
中文翻译:

电场催化的坎普消除反应。
肯普消除反应是新酶从头设计中使用最广泛的反应。从理论上研究了两种不同电场在乙酸根作为碱与苯并异恶唑和5-硝基苯并恶唑为底物的反应中的作用。已使用SMD连续模型对几种溶剂计算了溶剂反应场的影响。我们已经表明,溶剂抑制了两个反应,只要介电常数增加,反应速率的降低就更大。在非质子有机溶剂和质子溶剂如水之间,反应速率的降低尤为明显,氢键的静电项是水抑制作用大的主要因素。沿电荷转移方向(z轴)定向的外部电场的存在会增加电荷反应速率,从而提高反应速率。在硝基化合物的反应中,如果电场沿正交方向(x轴)取向,则电荷转移到NO2组是有利的,并且随后反应速率增加。但是,这种增加小于由z轴上的磁场产生的增加。值得一提的是,中等强度外部电场的主要作用之一是反应物的重新定向。最后,讨论了我们的结果在酶从头设计中的意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号