当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tuning the Competition between Hydrogen and Tetrel Bonds by a Magnesium Bond.
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-01-10 , DOI: 10.1002/cphc.201901076 Mingchang Hou 1 , Yifan Zhu 1 , Qingzhong Li 1 , Steve Scheiner 2
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-01-10 , DOI: 10.1002/cphc.201901076 Mingchang Hou 1 , Yifan Zhu 1 , Qingzhong Li 1 , Steve Scheiner 2
Affiliation
|
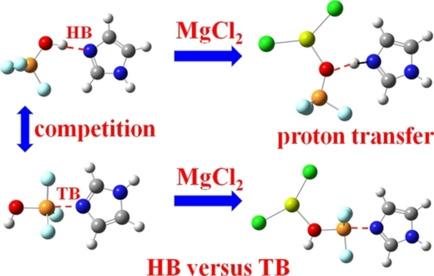
A computational study of the complexes formed by TF3OH (T=C, Si, Ge) with three nitrogen‐containing bases NCH, NH3, and imidazole (IM) is carried out at the MP2/aug‐cc‐pVTZ level. TF3OH can participate in two different types of noncovalent interactions: a hydrogen bond (HB) involving the hydroxyl proton and a tetrel bond (TB) with the tetel atom T. The strength of the HB is largely unaffected by the identity of T while the TB is enhanced as T grows larger. The HB is preferred over the TB for most systems, with the exception of GeF3OH with either NH3 or IM. MgCl2 engages in a Mg⋅⋅⋅O Magnesium bond (Mg‐bond) with the TF3OH O atom, which cooperatively enhances both the HB and TB. The HB strengthening is particularly large for the NH3 or IM bases, and especially for CF3OH, but is slowly reduced as the T atom grows larger. The TB enhancement, on the other hand, behaves in the opposite fashion, accelerating for the larger T atoms. As a bottom line, the Mg‐bond generally reinforces and accentuates the preference for the HB or TB that is already present in the dimer. The Mg‐bond is also responsible for a proton transfer in the HB configurations with NH3 and IM.
中文翻译:

通过镁键调节氢键和四氢键之间的竞争。
在MP2 / aug-cc-pVTZ水平上进行了由TF 3 OH(T = C,Si,Ge)与三种含氮碱NCH,NH 3和咪唑(IM)形成的络合物的计算研究。TF 3 OH可以参与两种不同类型的非共价相互作用:涉及羟基质子的氢键(HB)和与teltel原子T的蝶形键(TB)。HB的强度在很大程度上不受T的身份的影响随着T的增大,TB会增强。在大多数系统中,HB比TB更可取,除了GeF 3 OH和NH 3或IM以外。MgCl 2与TF 3发生Mg⋅⋅⋅O镁键(Mg-bond)OH O原子,可协同增强HB和TB。对于NH 3或IM碱,尤其是对于CF 3 OH,HB的增强特别大,但随着T原子的增大,HB的增强缓慢降低。另一方面,TB增强以相反的方式表现,对于较大的T原子加速。作为底线,镁键通常会增强并加强对二聚体中已存在的HB或TB的偏爱。镁键还负责在NH 3和IM的HB配置中进行质子转移。
更新日期:2020-01-10
中文翻译:

通过镁键调节氢键和四氢键之间的竞争。
在MP2 / aug-cc-pVTZ水平上进行了由TF 3 OH(T = C,Si,Ge)与三种含氮碱NCH,NH 3和咪唑(IM)形成的络合物的计算研究。TF 3 OH可以参与两种不同类型的非共价相互作用:涉及羟基质子的氢键(HB)和与teltel原子T的蝶形键(TB)。HB的强度在很大程度上不受T的身份的影响随着T的增大,TB会增强。在大多数系统中,HB比TB更可取,除了GeF 3 OH和NH 3或IM以外。MgCl 2与TF 3发生Mg⋅⋅⋅O镁键(Mg-bond)OH O原子,可协同增强HB和TB。对于NH 3或IM碱,尤其是对于CF 3 OH,HB的增强特别大,但随着T原子的增大,HB的增强缓慢降低。另一方面,TB增强以相反的方式表现,对于较大的T原子加速。作为底线,镁键通常会增强并加强对二聚体中已存在的HB或TB的偏爱。镁键还负责在NH 3和IM的HB配置中进行质子转移。


























 京公网安备 11010802027423号
京公网安备 11010802027423号