当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhanced hydrogen generation performance of CaMg2-based materials by ball milling
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2019/12/23 , DOI: 10.1039/c9qi01299k Miaolian Ma 1, 2, 3, 4, 5 , Kang Chen 4, 5, 6, 7, 8 , Jun Jiang 4, 5, 6, 7, 8 , Xusheng Yang 4, 9, 10, 11, 12 , Hui Wang 4, 5, 6, 7, 8 , Huaiyu Shao 4, 13, 14, 15, 16 , Jiangwen Liu 4, 5, 6, 7, 8 , Liuzhang Ouyang 4, 5, 6, 7, 8
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2019/12/23 , DOI: 10.1039/c9qi01299k Miaolian Ma 1, 2, 3, 4, 5 , Kang Chen 4, 5, 6, 7, 8 , Jun Jiang 4, 5, 6, 7, 8 , Xusheng Yang 4, 9, 10, 11, 12 , Hui Wang 4, 5, 6, 7, 8 , Huaiyu Shao 4, 13, 14, 15, 16 , Jiangwen Liu 4, 5, 6, 7, 8 , Liuzhang Ouyang 4, 5, 6, 7, 8
Affiliation
|
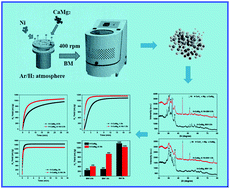
Non-catalytic hydrolysis of CaMg2-based materials (abbreviated as CaMg2, CaMg2–0.1Ni, H-CaMg2, and H-CaMg2–0.1Ni hereinafter) fabricated by ball milling for hydrogen supply has been investigated in the present work. With respect to the as-melted counterparts, it is found that both milled CaMg2 and H-CaMg2-based samples can significantly enhance the hydrolysis performance via adjusting the milling durations. In particular, 0.5 h-milled CaMg2 and 3 h-milled H-CaMg2 exert optimal kinetics at ambient temperature, delivering a hydrogen yield of 539 mL g−1 within 2 h and 1439 mL g−1 of H2 within only 3 min, respectively. In addition, the further results indicate that the hydrogen uptake of CaMg2 can be accelerated by doping with the Ni element, giving rise to considerably enhanced hydrolytic dynamics, as opposed to a limited promotion of the hydrolysis of the CaMg2 alloy. For example, the hydrogen yield of H-CaMg2–0.1Ni increases from 853 to 1147 mL g−1 H2 in 5 min with hydrogenation durations ranging from 0.5 to 1.5 h, much higher than the values (598–954 mL g−1 H2) of H-CaMg2 under the same conditions. More specifically, the 3 h-milled H-CaMg2 sample also demonstrates excellent cryogenic hydrolysis kinetics, achieving a hydrogen yield of 1332 mL g−1 H2 within 15 min at 0 °C. In comparison with the conventional hydrogenation of pristine CaMg2 conducted at elevated temperature, a more feasible strategy is applied to realize its hydrogen uptake by ball milling with Ni under mild conditions. Expectedly, the hydrogen supply capacities of the hydrogenated samples are markedly enhanced, making them promising to achieve their wide applications in hydrogen energy areas in the future.
中文翻译:

球磨增强了CaMg2基材料的制氢性能
目前,已经研究了通过球磨制备的CaMg 2基材料(以下简称为CaMg 2,CaMg 2 –0.1Ni,H-CaMg 2和H-CaMg 2 –0.1Ni)的非催化水解以供氢。工作。关于作为熔化的对应,可以发现,无论研磨为CaMg 2和H-为CaMg 2米系的样品可以显著提高水解性能通过调整研磨持续时间。特别是,在环境温度下0.5 h研磨的CaMg 2和3 h研磨的H-CaMg 2表现出最佳的动力学,提供539 mL g -1的氢气产率仅在3小时内分别在2 h和1439 mL g -1的H 2中。此外,进一步的结果表明,与有限地促进CaMg 2合金的水解相反,通过掺杂Ni元素可以促进CaMg 2的氢吸收,从而大大提高了水解动力学。例如,H-为CaMg的氢产率2个从853 -0.1Ni增加至1147毫升克-1 ħ 2在5分钟内用氢化持续时间范围从0.5到1.5小时,低于值(598-954毫升克高得多- 1 H 2)的H-CaMg 2在相同条件下。更具体地说,经过3 h研磨的H-CaMg 2样品还显示出出色的低温水解动力学,在0°C下15分钟内获得了1332 mL g -1 H 2的氢产率。与在高温下进行的传统原始CaMg 2加氢相比,采用了一种更可行的策略,以在温和的条件下用Ni进行球磨来实现其对氢的吸收。可以预期,氢化样品的氢供应能力将显着提高,使其有望在将来在氢能领域获得广泛的应用。
更新日期:2020-02-18
中文翻译:

球磨增强了CaMg2基材料的制氢性能
目前,已经研究了通过球磨制备的CaMg 2基材料(以下简称为CaMg 2,CaMg 2 –0.1Ni,H-CaMg 2和H-CaMg 2 –0.1Ni)的非催化水解以供氢。工作。关于作为熔化的对应,可以发现,无论研磨为CaMg 2和H-为CaMg 2米系的样品可以显著提高水解性能通过调整研磨持续时间。特别是,在环境温度下0.5 h研磨的CaMg 2和3 h研磨的H-CaMg 2表现出最佳的动力学,提供539 mL g -1的氢气产率仅在3小时内分别在2 h和1439 mL g -1的H 2中。此外,进一步的结果表明,与有限地促进CaMg 2合金的水解相反,通过掺杂Ni元素可以促进CaMg 2的氢吸收,从而大大提高了水解动力学。例如,H-为CaMg的氢产率2个从853 -0.1Ni增加至1147毫升克-1 ħ 2在5分钟内用氢化持续时间范围从0.5到1.5小时,低于值(598-954毫升克高得多- 1 H 2)的H-CaMg 2在相同条件下。更具体地说,经过3 h研磨的H-CaMg 2样品还显示出出色的低温水解动力学,在0°C下15分钟内获得了1332 mL g -1 H 2的氢产率。与在高温下进行的传统原始CaMg 2加氢相比,采用了一种更可行的策略,以在温和的条件下用Ni进行球磨来实现其对氢的吸收。可以预期,氢化样品的氢供应能力将显着提高,使其有望在将来在氢能领域获得广泛的应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号