当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unusual behaviour of the spin-spin coupling constant 1JCH upon formation of CHX hydrogen bond.
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-01-09 , DOI: 10.1039/c9cp05964d Elena Yu Tupikina 1 , Gleb S Denisov 2 , Alexander S Antonov 1 , Peter M Tolstoy 1
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-01-09 , DOI: 10.1039/c9cp05964d Elena Yu Tupikina 1 , Gleb S Denisov 2 , Alexander S Antonov 1 , Peter M Tolstoy 1
Affiliation
|
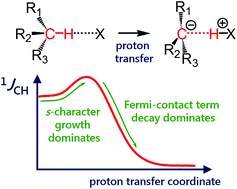
One-bond coupling constants 1JXY are usually used as a measure of the corresponding XY interatomic distances. However, the physical nature of this correlation is not well understood and, in some cases, a counterintuitive behaviour of 1JXY upon hydrogen bonded complex formation has been reported. In this work, the behavior of 1JCH upon formation and strengthening of complexes with CHX hydrogen bonds and upon a proton transfer process is investigated by means of 1H NMR spectroscopy and quantum chemical calculations. 1H NMR spectra of 1,1-dinitroethane solution at room temperature in various solvents (carbon tetrachloride, chloroform, dichloromethane, acetone, dimethylformamide and dimethyl sulfoxide) illustrate the increase of 1JCH by several Hz upon an increase of the complex strength. Computational results (MP2/aug-cc-pVDZ) reproduce this observation and allow one to conclude that the increase of 1JCH is mainly caused by the change of the carbon hybridization (an increase of s-character), rather than by the change in interatomic distance rCH. The behavior of 1JCH was also examined computationally for a wide range of CHX hydrogen bond energies and geometries. For this purpose, quantum-chemical modeling of the partial proton transfer process for complexes formed by 1,1-dinitroethane and trinitromethane as hydrogen bond donors with acetone, pyridine and fluoride anion as hydrogen bond acceptors was performed. The obtained results have confirmed the above-mentioned idea - for rather weak complexes, the dominant impact on the change of 1JCH magnitude is the increase of the s-character of carbon atom hybridization, while for complexes with a significantly transferred proton, the exponential decrease of the Fermi-contact term dominates.
中文翻译:

形成CHX氢键时自旋-自旋偶联常数1JCH的异常行为。
单键耦合常数1JXY通常用作相应XY原子间距离的量度。但是,这种相关性的物理性质尚未得到很好的理解,并且在某些情况下,已经报道了1JXY在形成氢键配合物时的反直觉行为。在这项工作中,通过1H NMR光谱和量子化学计算研究了1JCH在形成和增强具有CHX氢键的配合物时以及在质子转移过程中的行为。室温下1,1-二硝基乙烷溶液在各种溶剂(四氯化碳,氯仿,二氯甲烷,丙酮,二甲基甲酰胺和二甲基亚砜)中的1H NMR光谱表明,随着络合物强度的增加,1JCH的增加数Hz。计算结果(MP2 / aug-cc-pVDZ)重现了这一观察结果,可以得出一个结论,即1JCH的增加主要是由于碳杂化的变化(s字符的增加)引起的,而不是由于原子间的变化引起的。距离rCH。还对各种CHX氢键能量和几何结构进行了1JCH的行为计算研究。为此,对由1,1-二硝基乙烷和三硝基甲烷作为氢键供体,以丙酮,吡啶和氟阴离子作为氢键受体形成的配合物进行了部分质子转移过程的量子化学建模。获得的结果证实了上述想法-对于较弱的配合物,对1JCH幅度变化的主要影响是碳原子杂交的s-字符的增加,
更新日期:2020-01-09
中文翻译:

形成CHX氢键时自旋-自旋偶联常数1JCH的异常行为。
单键耦合常数1JXY通常用作相应XY原子间距离的量度。但是,这种相关性的物理性质尚未得到很好的理解,并且在某些情况下,已经报道了1JXY在形成氢键配合物时的反直觉行为。在这项工作中,通过1H NMR光谱和量子化学计算研究了1JCH在形成和增强具有CHX氢键的配合物时以及在质子转移过程中的行为。室温下1,1-二硝基乙烷溶液在各种溶剂(四氯化碳,氯仿,二氯甲烷,丙酮,二甲基甲酰胺和二甲基亚砜)中的1H NMR光谱表明,随着络合物强度的增加,1JCH的增加数Hz。计算结果(MP2 / aug-cc-pVDZ)重现了这一观察结果,可以得出一个结论,即1JCH的增加主要是由于碳杂化的变化(s字符的增加)引起的,而不是由于原子间的变化引起的。距离rCH。还对各种CHX氢键能量和几何结构进行了1JCH的行为计算研究。为此,对由1,1-二硝基乙烷和三硝基甲烷作为氢键供体,以丙酮,吡啶和氟阴离子作为氢键受体形成的配合物进行了部分质子转移过程的量子化学建模。获得的结果证实了上述想法-对于较弱的配合物,对1JCH幅度变化的主要影响是碳原子杂交的s-字符的增加,



























 京公网安备 11010802027423号
京公网安备 11010802027423号