当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Correlation between mobility and the hydrogen bonding network of water at an electrified-graphite electrode using molecular dynamics simulation.
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-01-03 , DOI: 10.1039/c9cp06013h Masaya Imai 1 , Yasuyuki Yokota 2 , Ichiro Tanabe 1 , Kouji Inagaki 3 , Yoshitada Morikawa 4 , Ken-Ichi Fukui 5
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-01-03 , DOI: 10.1039/c9cp06013h Masaya Imai 1 , Yasuyuki Yokota 2 , Ichiro Tanabe 1 , Kouji Inagaki 3 , Yoshitada Morikawa 4 , Ken-Ichi Fukui 5
Affiliation
|
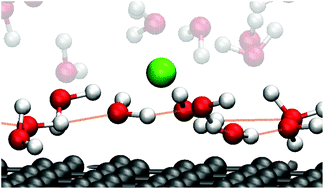
Focusing on the electric double layer formed at aqueous solution/graphite electrode interfaces, we investigated the relationship between the mobility of interfacial water and its hydrogen bonding networks by using molecular dynamics simulations. We focused on the mobility of the first hydration layer constructed nearest to the electrode. The mobility was determined by calculating the diffusion coefficient which showed an opposite trend to that of the applied potential polarity. The mobility decreased upon positive potentials while showing an increase upon negative potentials, which is rationalized by the strength of the interfacial hydrogen bonding networks.
中文翻译:

分子动力学模拟在带电石墨电极上水的迁移率与氢键网络之间的相关性。
着眼于水溶液/石墨电极界面处形成的双电层,我们使用分子动力学模拟研究了界面水的迁移率与其氢键网络之间的关系。我们专注于最靠近电极的第一水合层的迁移率。通过计算扩散系数来确定迁移率,该扩散系数显示出与所施加的电位极性相反的趋势。迁移率在正电势下降低,而在负电势上却增加,这由界面氢键网络的强度合理化。
更新日期:2020-01-08
中文翻译:

分子动力学模拟在带电石墨电极上水的迁移率与氢键网络之间的相关性。
着眼于水溶液/石墨电极界面处形成的双电层,我们使用分子动力学模拟研究了界面水的迁移率与其氢键网络之间的关系。我们专注于最靠近电极的第一水合层的迁移率。通过计算扩散系数来确定迁移率,该扩散系数显示出与所施加的电位极性相反的趋势。迁移率在正电势下降低,而在负电势上却增加,这由界面氢键网络的强度合理化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号