当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermodynamic Properties for Aqueous MgB4O7 Solution at 298.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2019-12-23 , DOI: 10.1021/acs.jced.9b00663 Haiwen Ge 1, 2 , Min Wang 1, 2 , Yan Yao 1, 2 , Tianlong Deng 3
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2019-12-23 , DOI: 10.1021/acs.jced.9b00663 Haiwen Ge 1, 2 , Min Wang 1, 2 , Yan Yao 1, 2 , Tianlong Deng 3
Affiliation
|
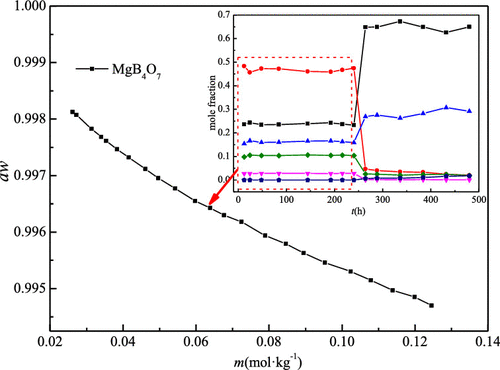
The solubility and behavior of phase transformation for MgB4O7 + H2O were studied at 298.15 K by the method of isothermal dissolution equilibrium. The results indicated that the equilibrium solid phase MgB4O7·9H2O was kept in the aqueous solution for 240 h and started to transform into inderite (MgB3O3(OH)5·5H2O) after 240 h. The concentration of the equilibrium liquid phase with respect to MgB4O7·9H2O is 0.03494 mol·kg–1 as metastable solubility. The isopiestic solubility, the osmotic coefficient, and water activity for the saturated solution of MgB4O7·9H2O at 298.15 ± 0.01 K were determined by the isopiestic method first, and obtained the values of 0.03536 mol·kg–1, 1.8753, and 0.99761, respectively. The Pitzer’s ion-interaction parameters and the equilibrium constant ln K for the MgB4O7 + H2O system at 298.15 K were obtained by fitting the experimental data of osmotic coefficients and the measurements of solubility and activity properties by the isopiestic method using the Pitzer equation of the osmotic coefficient with an adjusted α2 and the equilibrium constant equation simultaneously. The standard deviation is 0.005391 for the fit. The solubility of MgB4O7·9H2O was calculated using the parameterized equations and the lnK of 0.71826. The calculated isopiestic solubility is 0.03530 mol·kg–1, which agrees well with isothermal solubility and experimental isopiestic solubility.
中文翻译:

MgB 4 O 7水溶液在298.15 K时的热力学性质
采用等温溶解平衡法研究了MgB 4 O 7 + H 2 O在298.15 K时的溶解度和相变行为。结果表明,平衡固相MgB 4 O 7 ·9H 2 O在水溶液中保持240 h后,在240 h后开始转变为菱铁矿(MgB 3 O 3(OH)5 ·5H 2 O)。平衡液相相对于MgB 4 O 7 ·9H 2 O的浓度为0.03494 mol·kg –1作为亚稳溶解度。所述等压溶解度,渗透系数,和水活度为硼化镁的饱和溶液4 ö 7 ·9H 2 O不连到298.15±0.01 K含量通过等压法首先确定,并获得了0.03536摩尔·千克的值-1,1.8753和0.99761。MgB 4 O 7 + H 2 O体系在298.15 K时的Pitzer离子相互作用参数和平衡常数ln K是通过对渗透系数的实验数据进行拟合,并用等离子方法测定溶解度和活性性质而得到的。调整后的α的渗透系数的Pitzer方程2和平衡常数方程同时进行。拟合的标准偏差为0.005391。MgB 4 O 7 ·9H 2 O的溶解度通过参数公式计算得出,ln K为0.71826。计算得出的等渗溶解度为0.03530 mol·kg –1,与等温溶解度和实验等渗溶解度非常吻合。
更新日期:2019-12-23
中文翻译:

MgB 4 O 7水溶液在298.15 K时的热力学性质
采用等温溶解平衡法研究了MgB 4 O 7 + H 2 O在298.15 K时的溶解度和相变行为。结果表明,平衡固相MgB 4 O 7 ·9H 2 O在水溶液中保持240 h后,在240 h后开始转变为菱铁矿(MgB 3 O 3(OH)5 ·5H 2 O)。平衡液相相对于MgB 4 O 7 ·9H 2 O的浓度为0.03494 mol·kg –1作为亚稳溶解度。所述等压溶解度,渗透系数,和水活度为硼化镁的饱和溶液4 ö 7 ·9H 2 O不连到298.15±0.01 K含量通过等压法首先确定,并获得了0.03536摩尔·千克的值-1,1.8753和0.99761。MgB 4 O 7 + H 2 O体系在298.15 K时的Pitzer离子相互作用参数和平衡常数ln K是通过对渗透系数的实验数据进行拟合,并用等离子方法测定溶解度和活性性质而得到的。调整后的α的渗透系数的Pitzer方程2和平衡常数方程同时进行。拟合的标准偏差为0.005391。MgB 4 O 7 ·9H 2 O的溶解度通过参数公式计算得出,ln K为0.71826。计算得出的等渗溶解度为0.03530 mol·kg –1,与等温溶解度和实验等渗溶解度非常吻合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号