当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Understanding the Role of Metallocenium Ion-Pair Aggregates on the Rate of Olefin Insertion into the Metal–Carbon Bond
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-01-08 , DOI: 10.1021/acscatal.9b04929 Leonardo Sian 1, 2 , Alceo Macchioni 1, 2 , Cristiano Zuccaccia 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-01-08 , DOI: 10.1021/acscatal.9b04929 Leonardo Sian 1, 2 , Alceo Macchioni 1, 2 , Cristiano Zuccaccia 1, 2
Affiliation
|
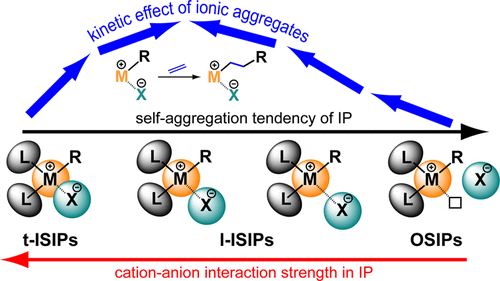
An integrated thermodynamic and kinetic study was carried out to clarify how the formation of ionic aggregates higher than ion pairs affects the rate of olefin insertion into the Zr–C bond. To this aim, the reaction with olefin of three-member zirconaziridium metallacycles [Cp2Zr(η2-CH2-N(R)2)]+[X]− [R = C18H37; X– = MeB(C6F5)3– (1BN) or B(C6F5)4– (1BT)], models for tight (t-ISIP, 1BN) and loose (l-ISIP, 1BT) inner sphere ion pair (ISIPs), was studied at different levels of concentration and thus self-aggregation. Both 1BN and 1BT undergo a regioselective insertion of a single α-olefin molecule (1-hexene or 2-Me-1-heptene) into the Zr–C bond to form the corresponding five-member metallacycles outer sphere ion pairs (2BN, 2BT, 3BN, and 3BT), with no evidence for further olefin insertions. Under comparable experimental conditions, where monomeric ion pairs dominate (aggregation number N ≈ 1), the apparent rate constant (kapp) of 1-hexene insertion into 1BT is about 200 times higher than that in 1BN, in agreement with the weaker strength of cation–anion interaction in the former. For 1BT, kapp is only marginally affected by the self-aggregation level in all investigated experimental conditions. For example, kapp = 1.1 M–1 s–1 (N ≈ 1.1) and 1.3 (N ≈ 4.8) for the insertion reaction of 1-hexene in toluene at 223 K and range from 19 × 10–3 M–1 s–1 (N ≈ 1.5) to 10 × 10–3 (N ≈ 39) for the insertion of 2-Me-1-heptene in methylcyclohexane at 258 K. On the contrary, kapp is markedly dependent on self-aggregation in the case of 1BN. For instance, when 1BN is reacted with 1-hexene in methylcyclohexane at 258 K, kapp increases more than 1 order of magnitude, from 6 × 10–3 to 93 × 10–3 M–1 s–1, upon increasing the aggregation level from N ≈ 1 ([1BN] = 6 × 10–5 M) to N ≈ 4.6 ([1BN] = 12.8 × 10–3 M). Although the insertion of 1-hexene in 1BN is faster in the slightly more polar solvent toluene (kapp = 39 × 10–5 M, N ≈ 1), the relative reactivity difference between ion pairs and larger ionic aggregates is similar in both solvents (about 4× and 3× increment, on passing from N ≈ 1.0 to N ≈ 1.6, in methylcyclohexane and toluene, respectively). The addition of an inert external salt with a common counterion (2BN) to solution of 1BN dramatically raises the olefin insertion rate constant: at [1BN] = 6 × 10–5 M, kapp increases from 6 × 10–3 M–1 s–1, in the absence of 2BN, to 42 × 10–3, 90 × 10–3, and 166 × 10–3 M–1 s–1 in the presence of 1, 3, and 5 equiv of 2BN, respectively. All of the thermodynamic and kinetic data can be rationalized by taking into account the strength of cation–anion interaction in the two kinds of ion pairs. In t-ISIPs, the cation–anion interaction energy is relatively strong, resulting in a relatively small dipole moment and a little thermodynamic tendency to self-aggregate. However, under conditions where homo (or mixed) ion aggregates form, an additional weakening of the cation–anion interaction occurs, causing a substantial increase of the intrinsic reactivity of each t-ISIP within aggregates. On the other side, the cation–anion interaction is already relatively weak in l-ISIPs and ionic aggregates forms much more easily from the thermodynamic point of view; in this case, however, the extra weakening of cation–anion interaction only marginally alters the overall barrier for olefin insertion in each l-ISIP.
中文翻译:

了解茂金属离子对聚集体对烯烃插入金属-碳键的速率的作用
进行了综合的热力学和动力学研究,以阐明高于离子对的离子聚集体的形成如何影响烯烃插入Zr-C键的速率。为了达到这个目的,用三构件zirconaziridium metallacycles烯烃反应的[Cp 2 Zr的(η 2 -CH 2 -N(R)2)] + [X] - [R = C 18 H ^ 37 ; X – = MeB(C 6 F 5)3 –(1BN)或B(C 6 F 5)4 –(1BT)],研究了不同浓度和自聚集条件下紧密(t-ISIP,1BN)和宽松(l-ISIP,1BT)内球离子对(ISIPs)的模型。既1BN和1BT经历单α烯烃分子的区域选择性插入(1-己烯或2-ME -1-庚烯)插入的Zr-C键以形成相应的五构件metallacycles外球离子对(2BN,2BT,3BN和3BT),没有进一步插入烯烃的证据。下可比试验条件下,其中的单体离子对支配(聚集数Ñ ≈1),表观速率常数(ķ应用)1-己烯在1BT中的插入量比1BN中高200倍左右,这与前者中阳离子-阴离子相互作用的强度较弱有关。对于1BT,在所有研究的实验条件下,k app仅受自身聚集水平的影响很小。例如,ķ应用程式= 1.1M,-1小号-1(Ñ ≈1.1)和1.3(Ñ ≈4.8)在223 K的甲苯1-己烯的插入反应和范围从19×10个-3中号-1小号-1(ñ ≈1.5)〜10×10 -3(Ñ ≈39),用于在258 K. 2-ME -1-庚烯在甲基环己烷中相反的插入,ķ应用是显着地依赖于自聚集在的情况下1BN。例如,当1BN与1-己烯在258 K的甲基环己烷中反应时,随着团聚的增加,k app会增加1个数量级,从6×10 –3增至93×10 –3 M –1 s –1。电平从ñ ≈1([ 1BN ] = 6×10 -5 M)以ñ ≈4.6([ 1BN ] = 12.8×10 -3M)。尽管在极性稍强的甲苯中(1- kN = 1× N)中1-己烯在1BN中的插入速度更快(k app = 39×10 –5 M,N≈1),但两种溶剂中离子对和较大的离子聚集体之间的相对反应性差异相似(约4×3×增量,从传递ñ ≈1.0〜ñ ≈1.6,在甲基环己烷和甲苯,分别地)。在1BN溶液中添加具有共同抗衡离子(2BN)的惰性外盐会显着提高烯烃的插入速率常数:[ 1BN ] = 6×10 –5 M,k app从6×10增加-3中号-1小号-1,在没有2BN,到42×10 -3,90×10 -3,和166×10 -3中号-1小号-1中的1的存在,3和5当量的2BN, 分别。通过考虑两种离子对中阳离子与阴离子相互作用的强度,可以合理化所有的热力学和动力学数据。在t-ISIPs中,阳离子与阴离子的相互作用能相对较强,导致偶极矩较小,并且自聚集的热力学趋势较小。但是,在均质(或混合)离子聚集体形成的条件下,阳离子与阴离子之间的相互作用会进一步减弱,从而导致聚集体中每个t-ISIP的固有反应性大大提高。另一方面,在l-ISIPs中,阳离子与阴离子的相互作用已经相对较弱,从热力学的角度来看,离子聚集体的形成要容易得多。在这种情况下,
更新日期:2020-01-08
中文翻译:

了解茂金属离子对聚集体对烯烃插入金属-碳键的速率的作用
进行了综合的热力学和动力学研究,以阐明高于离子对的离子聚集体的形成如何影响烯烃插入Zr-C键的速率。为了达到这个目的,用三构件zirconaziridium metallacycles烯烃反应的[Cp 2 Zr的(η 2 -CH 2 -N(R)2)] + [X] - [R = C 18 H ^ 37 ; X – = MeB(C 6 F 5)3 –(1BN)或B(C 6 F 5)4 –(1BT)],研究了不同浓度和自聚集条件下紧密(t-ISIP,1BN)和宽松(l-ISIP,1BT)内球离子对(ISIPs)的模型。既1BN和1BT经历单α烯烃分子的区域选择性插入(1-己烯或2-ME -1-庚烯)插入的Zr-C键以形成相应的五构件metallacycles外球离子对(2BN,2BT,3BN和3BT),没有进一步插入烯烃的证据。下可比试验条件下,其中的单体离子对支配(聚集数Ñ ≈1),表观速率常数(ķ应用)1-己烯在1BT中的插入量比1BN中高200倍左右,这与前者中阳离子-阴离子相互作用的强度较弱有关。对于1BT,在所有研究的实验条件下,k app仅受自身聚集水平的影响很小。例如,ķ应用程式= 1.1M,-1小号-1(Ñ ≈1.1)和1.3(Ñ ≈4.8)在223 K的甲苯1-己烯的插入反应和范围从19×10个-3中号-1小号-1(ñ ≈1.5)〜10×10 -3(Ñ ≈39),用于在258 K. 2-ME -1-庚烯在甲基环己烷中相反的插入,ķ应用是显着地依赖于自聚集在的情况下1BN。例如,当1BN与1-己烯在258 K的甲基环己烷中反应时,随着团聚的增加,k app会增加1个数量级,从6×10 –3增至93×10 –3 M –1 s –1。电平从ñ ≈1([ 1BN ] = 6×10 -5 M)以ñ ≈4.6([ 1BN ] = 12.8×10 -3M)。尽管在极性稍强的甲苯中(1- kN = 1× N)中1-己烯在1BN中的插入速度更快(k app = 39×10 –5 M,N≈1),但两种溶剂中离子对和较大的离子聚集体之间的相对反应性差异相似(约4×3×增量,从传递ñ ≈1.0〜ñ ≈1.6,在甲基环己烷和甲苯,分别地)。在1BN溶液中添加具有共同抗衡离子(2BN)的惰性外盐会显着提高烯烃的插入速率常数:[ 1BN ] = 6×10 –5 M,k app从6×10增加-3中号-1小号-1,在没有2BN,到42×10 -3,90×10 -3,和166×10 -3中号-1小号-1中的1的存在,3和5当量的2BN, 分别。通过考虑两种离子对中阳离子与阴离子相互作用的强度,可以合理化所有的热力学和动力学数据。在t-ISIPs中,阳离子与阴离子的相互作用能相对较强,导致偶极矩较小,并且自聚集的热力学趋势较小。但是,在均质(或混合)离子聚集体形成的条件下,阳离子与阴离子之间的相互作用会进一步减弱,从而导致聚集体中每个t-ISIP的固有反应性大大提高。另一方面,在l-ISIPs中,阳离子与阴离子的相互作用已经相对较弱,从热力学的角度来看,离子聚集体的形成要容易得多。在这种情况下,


























 京公网安备 11010802027423号
京公网安备 11010802027423号