当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular Model for the Surface-Catalyzed Protein Self-Assembly.
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jpcb.9b10052 Yangang Pan 1 , Siddhartha Banerjee 1 , Karen Zagorski 1 , Luda S Shlyakhtenko 1 , Anatoly B Kolomeisky 2 , Yuri L Lyubchenko 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jpcb.9b10052 Yangang Pan 1 , Siddhartha Banerjee 1 , Karen Zagorski 1 , Luda S Shlyakhtenko 1 , Anatoly B Kolomeisky 2 , Yuri L Lyubchenko 1
Affiliation
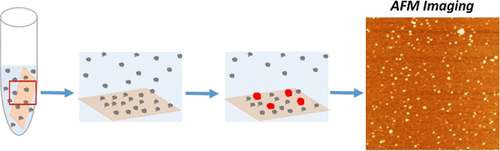
|
The importance of cell surfaces in the self-assembly of proteins is widely accepted. One biologically significant event is the assembly of amyloidogenic proteins into aggregates, which leads to neurodegenerative disorders like Alzheimer's and Parkinson's diseases. The interaction of amyloidogenic proteins with cellular membranes appears to dramatically facilitate the aggregation process. Recent findings indicate that, in the presence of surfaces, aggregation occurs at physiologically low concentrations, suggesting that interaction with surfaces plays a critical role in the disease-prone aggregation process. However, the molecular mechanisms behind the on-surface aggregation process remain unclear. Here, we provide a theoretical model that offers a molecular explanation. According to this model, monomers transiently immobilized to surfaces increase the local monomer protein concentration and thus work as nuclei to dramatically accelerate the entire aggregation process. This physical-chemical theory was verified by experimental studies, using mica surfaces, to examine the aggregation kinetics of amyloidogenic α-synuclein protein and non-amyloidogenic cytosine deaminase APOBEC3G.
中文翻译:

表面催化的蛋白质自组装的分子模型。
细胞表面在蛋白质自组装中的重要性已被广泛接受。一个生物学上重要的事件是淀粉样蛋白合成为聚集体,这会导致神经退行性疾病,例如阿尔茨海默氏病和帕金森氏病。淀粉样蛋白生成蛋白与细胞膜的相互作用似乎极大地促进了聚集过程。最近的发现表明,在存在表面的情况下,聚集发生在生理学上的低浓度下,这表明与表面的相互作用在易病性聚集过程中起着至关重要的作用。但是,表面聚集过程背后的分子机制仍不清楚。在这里,我们提供了一个理论模型,提供了分子解释。根据这个模型,暂时固定在表面上的单体增加了局部单体蛋白质的浓度,因此可以作为核来显着加速整个聚集过程。通过使用云母表面的实验研究验证了这种物理化学理论,以检查淀粉样蛋白生成的α-突触核蛋白和非淀粉样蛋白生成的胞嘧啶脱氨酶APOBEC3G的聚集动力学。
更新日期:2020-01-07
中文翻译:

表面催化的蛋白质自组装的分子模型。
细胞表面在蛋白质自组装中的重要性已被广泛接受。一个生物学上重要的事件是淀粉样蛋白合成为聚集体,这会导致神经退行性疾病,例如阿尔茨海默氏病和帕金森氏病。淀粉样蛋白生成蛋白与细胞膜的相互作用似乎极大地促进了聚集过程。最近的发现表明,在存在表面的情况下,聚集发生在生理学上的低浓度下,这表明与表面的相互作用在易病性聚集过程中起着至关重要的作用。但是,表面聚集过程背后的分子机制仍不清楚。在这里,我们提供了一个理论模型,提供了分子解释。根据这个模型,暂时固定在表面上的单体增加了局部单体蛋白质的浓度,因此可以作为核来显着加速整个聚集过程。通过使用云母表面的实验研究验证了这种物理化学理论,以检查淀粉样蛋白生成的α-突触核蛋白和非淀粉样蛋白生成的胞嘧啶脱氨酶APOBEC3G的聚集动力学。


























 京公网安备 11010802027423号
京公网安备 11010802027423号