当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iron‐Mediated Cyclization of 1,3‐Diynyl Propargyl Aryl Ethers with Dibutyl Diselenide: Synthesis of Selenophene‐Fused Chromenes
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-01-16 , DOI: 10.1002/adsc.201901410 Guilherme Lutz 1 , Davi F. Back 2 , Gilson Zeni 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-01-16 , DOI: 10.1002/adsc.201901410 Guilherme Lutz 1 , Davi F. Back 2 , Gilson Zeni 1
Affiliation
|
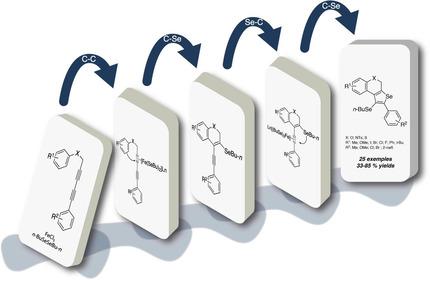
The synthesis of selenophene‐fused chromene derivatives starting from 1,3‐diynyl propargyl aryl ethers is reported herein. The method is based on carbon‐carbon, carbon‐selenium, selenium‐carbon and carbon‐selenium bonds formation in a one‐pot protocol, using iron(III) chloride and dibutyl diselenide as promoters. The same reaction conditions were applied to propargyl anilines leading to the formation of 1‐(butylselanyl)‐selenophene quinolines. The results showed that the dilution and temperature of substrate addition had a crucial influence in the products obtained. When the substrates were added at room temperature, in the absence of a solvent, a mixture of products was obtained, whereas the slowly addition (15 min) of starting materials, as a dichloromethane solution, at 0 °C led to the product formation in good yields. The mechanistic study indicates that the cooperative action between iron(III) chloride and dibutyl diselenide was essential to promote the cyclization, whereas separately none of them was effective in promoting the cyclization. We proved the synthetic utility of heterocycles obtained in the Suzuki cross coupling reaction, giving the corresponding cross‐coupled products in good yields. In addition, the organoselenium moiety was removed from the structures of products by using n‐butyllithium.
中文翻译:

铁与二丁基二硒化物的铁介导的1,3-二炔基炔丙基芳基醚的环化反应:硒化亚砜熔融二茂铁的合成
本文报道了从1,3-二炔基炔丙基芳基醚开始的硒亚砜稠合的亚甲基衍生物的合成。该方法基于一锅法中碳-碳,碳-硒,硒-碳和碳-硒键的形成,使用氯化铁(III)和二丁基二硒化物作为促进剂。将相同的反应条件应用于炔丙基苯胺,导致形成1-(丁基硒基)-硒苯醌喹啉。结果表明,底物添加的稀释度和温度对所得产物具有至关重要的影响。在室温下,在无溶剂的情况下添加底物时,会得到多种产物的混合物,而在二氯甲烷中于0°C缓慢添加(15分钟)起始原料则导致产物的形成。良品率高。机理研究表明,氯化铁(III)和二丁基二硒化物之间的协同作用对于促进环化是必不可少的,而它们中的任何一种都不能有效地促进环化。我们证明了在铃木交叉偶联反应中获得的杂环的合成效用,从而以高收率获得了相应的交叉偶联产物。此外,通过使用以下方法从产物结构中去除了有机硒部分正丁基锂。
更新日期:2020-01-17
中文翻译:

铁与二丁基二硒化物的铁介导的1,3-二炔基炔丙基芳基醚的环化反应:硒化亚砜熔融二茂铁的合成
本文报道了从1,3-二炔基炔丙基芳基醚开始的硒亚砜稠合的亚甲基衍生物的合成。该方法基于一锅法中碳-碳,碳-硒,硒-碳和碳-硒键的形成,使用氯化铁(III)和二丁基二硒化物作为促进剂。将相同的反应条件应用于炔丙基苯胺,导致形成1-(丁基硒基)-硒苯醌喹啉。结果表明,底物添加的稀释度和温度对所得产物具有至关重要的影响。在室温下,在无溶剂的情况下添加底物时,会得到多种产物的混合物,而在二氯甲烷中于0°C缓慢添加(15分钟)起始原料则导致产物的形成。良品率高。机理研究表明,氯化铁(III)和二丁基二硒化物之间的协同作用对于促进环化是必不可少的,而它们中的任何一种都不能有效地促进环化。我们证明了在铃木交叉偶联反应中获得的杂环的合成效用,从而以高收率获得了相应的交叉偶联产物。此外,通过使用以下方法从产物结构中去除了有机硒部分正丁基锂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号