当前位置:
X-MOL 学术
›
Process Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of a novel intestinal phospholipase A2 from annular seabream: Insights into its catalytic mechanism and its role in biological processes
Process Biochemistry ( IF 4.4 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.procbio.2019.12.012 Nabil Smichi , Houcemeddine Othman , Zied Zarai , Ahmed Fendri , Abdelkarim Abousalham
Process Biochemistry ( IF 4.4 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.procbio.2019.12.012 Nabil Smichi , Houcemeddine Othman , Zied Zarai , Ahmed Fendri , Abdelkarim Abousalham
|
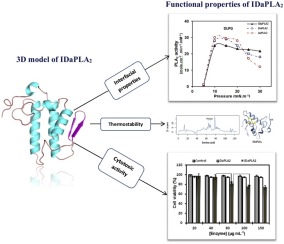
Abstract Phospholipase A2 (PLA2) is responsible for the lipid hydrolysis process. Fish PLA2 have warranted renewed interest due to their excellent properties in phospholipid digestion. We report for the first time the catalytic properties of a PLA2 secreted from the intestine of the annular seabream Diplodus annularis (IDaPLA2). The refolded IDaPLA2 was purified to homogeneity and showed a molecular mass of around 15 kDa attested by SDS-PAGE and MALDI-TOF analyses. Interestingly, IDaPLA2 revealed higher thermostability compared to mammal pancreatic sPLA2 as it was active and stable at 55 °C with specific activity of 290 U mg−1 on phosphatidylcholine (PC) as a substrate. Using the lipid monolayer technique, the activity of IDaPLA2 was found to be 21.68, 6.88 and 5.66 mol cm−2 min−1 mM−1 using phosphatidylglycerol (PG), PC and phosphatidylethanolamine (PE) monolayers, respectively, at surface pressures from 20−30 mN m−1. Interestingly, the interfacial activity of IDaPLA2 measured at higher surface pressures may highlight its ability to penetrate into phospholipid monolayers suggesting its involvement in cell lipid membrane degradation which can explain the cytotoxicity potential towards macrophage. The docking simulation data provided insights into the involvement of some key amino-acids in substrate binding and selectivity. The dynamic simulation proved the high stability of IDaPLA2. Overall, these results provide original evidence on the involvement of IDaPLA2 into the lipid hydrolysis suggesting it as a potential target in biotechnological applications.
中文翻译:

从环形鲷中鉴定新型肠磷脂酶 A2:对其催化机制及其在生物过程中的作用的洞察
摘要 磷脂酶 A2 (PLA2) 负责脂质水解过程。鱼类 PLA2 因其在磷脂消化方面的优异特性而重新引起人们的兴趣。我们首次报道了环状鲷鱼(IDaPLA2)肠道分泌的 PLA2 的催化特性。重折叠的 IDaPLA2 被纯化至同质,并显示出约 15 kDa 的分子量,通过 SDS-PAGE 和 MALDI-TOF 分析证明。有趣的是,与哺乳动物胰腺 sPLA2 相比,IDaPLA2 显示出更高的热稳定性,因为它在 55°C 下具有活性和稳定性,对作为底物的磷脂酰胆碱 (PC) 的比活性为 290 U mg-1。使用脂质单层技术,发现使用磷脂酰甘油 (PG) 的 IDaPLA2 的活性分别为 21.68、6.88 和 5.66 mol cm-2 min-1 mM-1,PC 和磷脂酰乙醇胺 (PE) 单层,分别在 20-30 mN m-1 的表面压力下。有趣的是,在较高表面压力下测量的 IDaPLA2 的界面活性可能突出其渗透到磷脂单层的能力,表明其参与细胞脂质膜降解,这可以解释对巨噬细胞的细胞毒性潜力。对接模拟数据提供了一些关键氨基酸参与底物结合和选择性的见解。动态模拟证明了IDaPLA2的高稳定性。总体而言,这些结果为 IDaPLA2 参与脂质水解提供了原始证据,表明它是生物技术应用中的潜在目标。在较高表面压力下测量的 IDaPLA2 的界面活性可能突出其渗透到磷脂单层的能力,表明其参与细胞脂质膜降解,这可以解释对巨噬细胞的细胞毒性潜力。对接模拟数据提供了一些关键氨基酸参与底物结合和选择性的见解。动态模拟证明了IDaPLA2的高稳定性。总体而言,这些结果为 IDaPLA2 参与脂质水解提供了原始证据,表明它是生物技术应用中的潜在目标。在较高表面压力下测量的 IDaPLA2 的界面活性可能突出其渗透到磷脂单层的能力,表明其参与细胞脂质膜降解,这可以解释对巨噬细胞的细胞毒性潜力。对接模拟数据提供了一些关键氨基酸参与底物结合和选择性的见解。动态模拟证明了IDaPLA2的高稳定性。总体而言,这些结果为 IDaPLA2 参与脂质水解提供了原始证据,表明它是生物技术应用中的潜在目标。
更新日期:2020-04-01
中文翻译:

从环形鲷中鉴定新型肠磷脂酶 A2:对其催化机制及其在生物过程中的作用的洞察
摘要 磷脂酶 A2 (PLA2) 负责脂质水解过程。鱼类 PLA2 因其在磷脂消化方面的优异特性而重新引起人们的兴趣。我们首次报道了环状鲷鱼(IDaPLA2)肠道分泌的 PLA2 的催化特性。重折叠的 IDaPLA2 被纯化至同质,并显示出约 15 kDa 的分子量,通过 SDS-PAGE 和 MALDI-TOF 分析证明。有趣的是,与哺乳动物胰腺 sPLA2 相比,IDaPLA2 显示出更高的热稳定性,因为它在 55°C 下具有活性和稳定性,对作为底物的磷脂酰胆碱 (PC) 的比活性为 290 U mg-1。使用脂质单层技术,发现使用磷脂酰甘油 (PG) 的 IDaPLA2 的活性分别为 21.68、6.88 和 5.66 mol cm-2 min-1 mM-1,PC 和磷脂酰乙醇胺 (PE) 单层,分别在 20-30 mN m-1 的表面压力下。有趣的是,在较高表面压力下测量的 IDaPLA2 的界面活性可能突出其渗透到磷脂单层的能力,表明其参与细胞脂质膜降解,这可以解释对巨噬细胞的细胞毒性潜力。对接模拟数据提供了一些关键氨基酸参与底物结合和选择性的见解。动态模拟证明了IDaPLA2的高稳定性。总体而言,这些结果为 IDaPLA2 参与脂质水解提供了原始证据,表明它是生物技术应用中的潜在目标。在较高表面压力下测量的 IDaPLA2 的界面活性可能突出其渗透到磷脂单层的能力,表明其参与细胞脂质膜降解,这可以解释对巨噬细胞的细胞毒性潜力。对接模拟数据提供了一些关键氨基酸参与底物结合和选择性的见解。动态模拟证明了IDaPLA2的高稳定性。总体而言,这些结果为 IDaPLA2 参与脂质水解提供了原始证据,表明它是生物技术应用中的潜在目标。在较高表面压力下测量的 IDaPLA2 的界面活性可能突出其渗透到磷脂单层的能力,表明其参与细胞脂质膜降解,这可以解释对巨噬细胞的细胞毒性潜力。对接模拟数据提供了一些关键氨基酸参与底物结合和选择性的见解。动态模拟证明了IDaPLA2的高稳定性。总体而言,这些结果为 IDaPLA2 参与脂质水解提供了原始证据,表明它是生物技术应用中的潜在目标。


























 京公网安备 11010802027423号
京公网安备 11010802027423号