当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
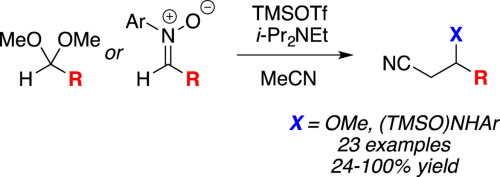
One-pot silyl ketene imine formation-nucleophilic addition reactions of acetonitrile with acetals and nitrones
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2019-12-20 , DOI: 10.1016/j.tetlet.2019.151537 C. Wade Downey , Grace Ann L. Robertson , Jhonmattew Santa , Kari R. Flicker , William M. Stith
中文翻译:

乙腈与乙缩醛和硝酮的一锅甲硅烷基乙烯酮亚胺形成亲核加成反应
更新日期:2019-12-21
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2019-12-20 , DOI: 10.1016/j.tetlet.2019.151537 C. Wade Downey , Grace Ann L. Robertson , Jhonmattew Santa , Kari R. Flicker , William M. Stith
|
Trimethylsilyl trifluoromethanesulfonate (TMSOTf) and a trialkylamine base promote the conversion of acetonitrile to its silyl ketene imine in situ when acetonitrile is employed as solvent. Residual TMSOTf acts as a Lewis acid catalyst to activate acetals and nitrones in the reaction mixture, yielding β-methoxynitriles and β -(silyloxy)aminonitriles, respectively. Some reaction products undergo elimination under the reaction conditions to provide the α, β -unsaturated nitrile directly.
中文翻译:

乙腈与乙缩醛和硝酮的一锅甲硅烷基乙烯酮亚胺形成亲核加成反应
当使用乙腈作为溶剂时,三氟甲磺酸三甲基甲硅烷基酯(TMSOTf)和三烷基胺碱可促进乙腈原位转化为其甲硅烷基烯酮亚胺。残留的TMSOTf充当路易斯酸催化剂,以活化反应混合物中的乙缩醛和硝酮,分别生成β-甲氧基腈和β-(甲硅烷氧基)氨基腈。一些反应产物在反应条件下被消除,以直接提供α,β-不饱和腈。


























 京公网安备 11010802027423号
京公网安备 11010802027423号