当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In situ hydroxyl radical generation using the synergism of the Co–Ni bimetallic centres of a developed nanocatalyst with potent efficiency for degrading toxic water pollutants
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2019/12/20 , DOI: 10.1039/c9qm00628a Rakesh K. Sharma 1, 2, 3, 4, 5 , Bhavya Arora 1, 2, 3, 4, 5 , Shivani Sharma 1, 2, 3, 4, 5 , Sriparna Dutta 1, 2, 3, 4, 5 , Aditi Sharma 1, 2, 3, 4, 5 , Sneha Yadav 1, 2, 3, 4, 5 , Kanika Solanki 1, 2, 3, 4, 5
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2019/12/20 , DOI: 10.1039/c9qm00628a Rakesh K. Sharma 1, 2, 3, 4, 5 , Bhavya Arora 1, 2, 3, 4, 5 , Shivani Sharma 1, 2, 3, 4, 5 , Sriparna Dutta 1, 2, 3, 4, 5 , Aditi Sharma 1, 2, 3, 4, 5 , Sneha Yadav 1, 2, 3, 4, 5 , Kanika Solanki 1, 2, 3, 4, 5
Affiliation
|
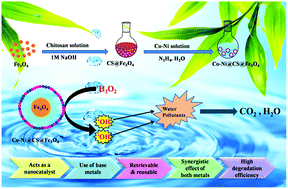
In today's global society, the development of novel materials for wastewater treatment has attracted the interest of academic and industrial researchers as this can ensure reliable access to clean water. Herein, we report for the first time the development of an economical and sustainable bimetallic nanocatalytic system comprising a polymeric magnetic chitosan support decorated with redox couple Co–Ni nanoparticles and highlight its role in the in situ generation of hydroxyl radical species that can effectively catalyze the degradation of toxic water contaminants, including the pesticide 2,4-dichlorophenoxyacetic acid (2,4-D) and the azo dye methyl orange (MO). Coumarin fluorescence probe and radical scavenging studies provided valuable insights into the mechanistic pathway, revealing the in situ generation of hydroxyl radicals that were responsible for expediting the degradation process. Kinetics studies revealed that the degradation process followed pseudo-first-order kinetics in both cases (with kinetic constant values of 0.07517 min−1 for 2,4-D and 0.03352 min−1 for MO at room temperature under neutral pH conditions). In addition, reusability studies suggested that the Co–Ni@CS@Fe3O4 nanocatalyst maintained its degradation efficacy even after eight consecutive cycles. Notably, this is the first report that effectively exploits a synergistic combination of two transition metals, Co and Ni, anchored onto the surface of chitosan-coated Fe3O4 nanoparticles to effectively catalyze the degradation of hazardous contaminants in the presence of a green oxidant under neutral and ambient conditions.
中文翻译:

利用已开发的纳米催化剂的Co-Ni双金属中心的协同作用原位产生羟基自由基,具有有效降解有毒水污染物的能力
在当今的全球社会中,用于废水处理的新型材料的开发吸引了学术和工业研究人员的兴趣,因为这可以确保可靠地获得清洁水。在此,我们首次报告了一种经济,可持续的双金属纳米催化系统的开发,该系统包括用氧化还原对Co-Ni纳米粒子修饰的聚合物磁性壳聚糖载体,并着重说明了其在可有效催化氢氧根自由基种类的原位生成中的作用。降解有毒的水污染物,包括农药2,4-二氯苯氧基乙酸(2,4-D)和偶氮染料甲基橙(MO)。香豆素荧光探针和自由基清除研究为机理研究提供了宝贵的见解,揭示了原位负责加速降解过程的羟基自由基的产生。动力学研究表明,在两种情况下,降解过程均遵循伪一级动力学(在中性pH条件下,室温下2,4-D的动力学常数为0.07517 min -1,MO的动力学常数为0.03352 min -1)。此外,可重复使用性研究表明,即使连续八个循环后,Co-Ni @ CS @ Fe 3 O 4纳米催化剂仍保持其降解功效。值得注意的是,这是第一份有效利用锚固在壳聚糖涂层的Fe 3 O 4表面上的两种过渡金属Co和Ni的协同组合的报告。 在绿色氧化剂存在下,在中性和环境条件下,纳米颗粒可有效催化有害污染物的降解。
更新日期:2020-02-13
中文翻译:

利用已开发的纳米催化剂的Co-Ni双金属中心的协同作用原位产生羟基自由基,具有有效降解有毒水污染物的能力
在当今的全球社会中,用于废水处理的新型材料的开发吸引了学术和工业研究人员的兴趣,因为这可以确保可靠地获得清洁水。在此,我们首次报告了一种经济,可持续的双金属纳米催化系统的开发,该系统包括用氧化还原对Co-Ni纳米粒子修饰的聚合物磁性壳聚糖载体,并着重说明了其在可有效催化氢氧根自由基种类的原位生成中的作用。降解有毒的水污染物,包括农药2,4-二氯苯氧基乙酸(2,4-D)和偶氮染料甲基橙(MO)。香豆素荧光探针和自由基清除研究为机理研究提供了宝贵的见解,揭示了原位负责加速降解过程的羟基自由基的产生。动力学研究表明,在两种情况下,降解过程均遵循伪一级动力学(在中性pH条件下,室温下2,4-D的动力学常数为0.07517 min -1,MO的动力学常数为0.03352 min -1)。此外,可重复使用性研究表明,即使连续八个循环后,Co-Ni @ CS @ Fe 3 O 4纳米催化剂仍保持其降解功效。值得注意的是,这是第一份有效利用锚固在壳聚糖涂层的Fe 3 O 4表面上的两种过渡金属Co和Ni的协同组合的报告。 在绿色氧化剂存在下,在中性和环境条件下,纳米颗粒可有效催化有害污染物的降解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号