当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of substituents on resonance assisted hydrogen bonding vs. intermolecular hydrogen bonding
CrystEngComm ( IF 3.1 ) Pub Date : 2019/12/20 , DOI: 10.1039/c9ce01744e Atash V. Gurbanov 1, 2, 3, 4, 5 , Maxim L. Kuznetsov 1, 2, 3, 4, 5 , Svetlana D. Demukhamedova 6, 7, 8, 9 , Irada N. Alieva 6, 7, 8, 9 , Niftali M. Godjaev 6, 7, 8, 9 , Fedor I. Zubkov 10, 11, 12, 13, 14 , Kamran T. Mahmudov 1, 2, 3, 4, 5 , Armando J. L. Pombeiro 1, 2, 3, 4, 5
CrystEngComm ( IF 3.1 ) Pub Date : 2019/12/20 , DOI: 10.1039/c9ce01744e Atash V. Gurbanov 1, 2, 3, 4, 5 , Maxim L. Kuznetsov 1, 2, 3, 4, 5 , Svetlana D. Demukhamedova 6, 7, 8, 9 , Irada N. Alieva 6, 7, 8, 9 , Niftali M. Godjaev 6, 7, 8, 9 , Fedor I. Zubkov 10, 11, 12, 13, 14 , Kamran T. Mahmudov 1, 2, 3, 4, 5 , Armando J. L. Pombeiro 1, 2, 3, 4, 5
Affiliation
|
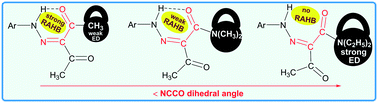
The role of substituents on resonance assisted hydrogen bonding (RAHB) vs. intermolecular hydrogen bonding is highlighted in known arylhydrazones of active methylene compounds (AHAMCs) and in their new representatives – (E/Z)-2-(2-(para-substitutedphenyl)hydrazineylidene)-N,N-diethyl-3-oxobutanamides (–C2H5, –H, –COOH, –CN). The strength of the H-bond depends on the attached functional groups to both the active methylene fragment and the aromatic moiety of the AHAMC. For instance, attachment of the strong electron donor group –N(C2H5)2 (σp = −0.72) to the active methylene fragment of AHAMC allows to isolate this class of compounds without RAHB but with strong intermolecular H-bonds, which are the first examples reported herein.
中文翻译:

取代基在共振辅助氢键与分子间氢键之间的作用
取代基在共振辅助氢键(RAHB)与分子间氢键上的作用在已知的活性亚甲基化合物(AHAMCs)芳基hydr及其新的代表体-(E / Z)-2-(2-(对-取代苯基)中得到强调)(亚肼基)-N,N-二乙基-3-氧代丁酰胺(–C 2 H 5,–H,–COOH,–CN)。H键的强度取决于与活性亚甲基片段和AHAMC的芳族部分连接的官能团。例如,强电子给体基团–N(C 2 H 5)2(σp= -0.72)至AHAMC的活性亚甲基片段允许分离出没有RAHB但具有强分子间H键的这类化合物,这是本文报道的第一个实例。
更新日期:2020-02-10
中文翻译:

取代基在共振辅助氢键与分子间氢键之间的作用
取代基在共振辅助氢键(RAHB)与分子间氢键上的作用在已知的活性亚甲基化合物(AHAMCs)芳基hydr及其新的代表体-(E / Z)-2-(2-(对-取代苯基)中得到强调)(亚肼基)-N,N-二乙基-3-氧代丁酰胺(–C 2 H 5,–H,–COOH,–CN)。H键的强度取决于与活性亚甲基片段和AHAMC的芳族部分连接的官能团。例如,强电子给体基团–N(C 2 H 5)2(σp= -0.72)至AHAMC的活性亚甲基片段允许分离出没有RAHB但具有强分子间H键的这类化合物,这是本文报道的第一个实例。



























 京公网安备 11010802027423号
京公网安备 11010802027423号