当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microwave assisted alkaline roasting-water leaching for the valorisation of goethite sludge from zinc refining process
Hydrometallurgy ( IF 4.7 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.hydromet.2019.105235 Thomas Abo Atia , Jeroen Spooren
Hydrometallurgy ( IF 4.7 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.hydromet.2019.105235 Thomas Abo Atia , Jeroen Spooren
|
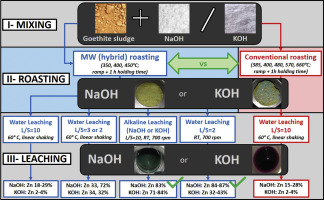
Abstract Microwave (MW) alkaline roasting followed by water leaching was studied to selectively extract valuable (Zn, Pb) and hazardous (As) elements for the decontamination of the goethite sludge. During alkaline roasting stable ZnFe2O4 was decomposed to ZnO. The influence of alkali source (KOH, NaOH) and the heating source (conventional vs. microwave) were systematically investigated. Generally, ZnFe2O4 decomposed better upon increasing roasting temperatures. For the water leaching step, different liquid-to-solid ratios (L:S = 10, 3, 2) and addition of alkaline agents (KOH, NaOH) were tested to enhance Zn extraction. NaOH addition to the leaching solution (60 °C, 30 min, L:S = 10) gave the best extractions for Zn and Pb for both MW KOH roasted (84.3 ± 0.4% Zn and 38 ± 3% Pb) and MW NaOH roasted (83 ± 1% Zn and 36.3 ± 0.3% Pb extraction) materials (400 °C, 60 min). Alternatively, decreasing the L:S of water leaching to 2 gave a similar Zn extraction (83 ± 1%) and reduced alkali consumption. By increasing the set MW roasting temperature to 450 °C, Zn leachability was 87 ± 3% (NaOH roasting, water leaching L:S = 2, 60 °C, 30 min). Additionally, As removal in the studied system was 98–100%. Interestingly, roasting with KOH increased the Mn solubility as (per)manganate and decreased Zn leachability by consuming OH– for disproportionation reactions of manganate. The leachability of matrix elements Ca and Fe was negligible.
中文翻译:

微波辅助碱性焙烧-水浸法用于锌精炼过程中针铁矿污泥的增值
摘要 研究了微波 (MW) 碱焙烧后水浸的选择性提取有价值 (Zn, Pb) 和有害 (As) 元素,用于针铁矿污泥的净化。在碱性焙烧过程中,稳定的 ZnFe2O4 被分解为 ZnO。系统地研究了碱源(KOH、NaOH)和加热源(常规与微波)的影响。一般来说,随着焙烧温度的升高,ZnFe2O4 分解得更好。对于水浸出步骤,测试了不同的液固比 (L:S = 10, 3, 2) 和添加碱性试剂 (KOH, NaOH) 以增强锌的提取。向浸出溶液中添加 NaOH(60 °C,30 分钟,L:S = 10)对 MW KOH 焙烧(84.3 ± 0.4% Zn 和 38 ± 3% Pb)和 MW NaOH 焙烧的 Zn 和 Pb 提供了最佳提取(83 ± 1% 锌和 36.3 ± 0。3% Pb 萃取)材料(400 °C,60 分钟)。或者,将水浸出的 L:S 降低到 2 可得到类似的锌提取率 (83 ± 1%) 并减少碱消耗。通过将设定的 MW 焙烧温度提高到 450 °C,锌浸出率为 87 ± 3%(NaOH 焙烧,水浸出 L:S = 2、60 °C、30 分钟)。此外,所研究系统中的砷去除率为 98-100%。有趣的是,用 KOH 焙烧增加了锰作为(过)锰酸盐的溶解度,并通过消耗 OH– 来进行锰酸盐的歧化反应,从而降低锌的浸出性。基质元素 Ca 和 Fe 的浸出性可以忽略不计。水浸出 L:S = 2, 60 °C, 30 min)。此外,所研究系统中的砷去除率为 98-100%。有趣的是,用 KOH 焙烧增加了 Mn 作为(过)锰酸盐的溶解度,并通过消耗 OH– 来进行锰酸盐的歧化反应,从而降低 Zn 的浸出性。基质元素 Ca 和 Fe 的浸出性可以忽略不计。水浸出 L:S = 2, 60 °C, 30 min)。此外,所研究系统中的砷去除率为 98-100%。有趣的是,用 KOH 焙烧增加了 Mn 作为(过)锰酸盐的溶解度,并通过消耗 OH– 来进行锰酸盐的歧化反应,从而降低 Zn 的浸出性。基质元素 Ca 和 Fe 的浸出性可以忽略不计。
更新日期:2020-01-01
中文翻译:

微波辅助碱性焙烧-水浸法用于锌精炼过程中针铁矿污泥的增值
摘要 研究了微波 (MW) 碱焙烧后水浸的选择性提取有价值 (Zn, Pb) 和有害 (As) 元素,用于针铁矿污泥的净化。在碱性焙烧过程中,稳定的 ZnFe2O4 被分解为 ZnO。系统地研究了碱源(KOH、NaOH)和加热源(常规与微波)的影响。一般来说,随着焙烧温度的升高,ZnFe2O4 分解得更好。对于水浸出步骤,测试了不同的液固比 (L:S = 10, 3, 2) 和添加碱性试剂 (KOH, NaOH) 以增强锌的提取。向浸出溶液中添加 NaOH(60 °C,30 分钟,L:S = 10)对 MW KOH 焙烧(84.3 ± 0.4% Zn 和 38 ± 3% Pb)和 MW NaOH 焙烧的 Zn 和 Pb 提供了最佳提取(83 ± 1% 锌和 36.3 ± 0。3% Pb 萃取)材料(400 °C,60 分钟)。或者,将水浸出的 L:S 降低到 2 可得到类似的锌提取率 (83 ± 1%) 并减少碱消耗。通过将设定的 MW 焙烧温度提高到 450 °C,锌浸出率为 87 ± 3%(NaOH 焙烧,水浸出 L:S = 2、60 °C、30 分钟)。此外,所研究系统中的砷去除率为 98-100%。有趣的是,用 KOH 焙烧增加了锰作为(过)锰酸盐的溶解度,并通过消耗 OH– 来进行锰酸盐的歧化反应,从而降低锌的浸出性。基质元素 Ca 和 Fe 的浸出性可以忽略不计。水浸出 L:S = 2, 60 °C, 30 min)。此外,所研究系统中的砷去除率为 98-100%。有趣的是,用 KOH 焙烧增加了 Mn 作为(过)锰酸盐的溶解度,并通过消耗 OH– 来进行锰酸盐的歧化反应,从而降低 Zn 的浸出性。基质元素 Ca 和 Fe 的浸出性可以忽略不计。水浸出 L:S = 2, 60 °C, 30 min)。此外,所研究系统中的砷去除率为 98-100%。有趣的是,用 KOH 焙烧增加了 Mn 作为(过)锰酸盐的溶解度,并通过消耗 OH– 来进行锰酸盐的歧化反应,从而降低 Zn 的浸出性。基质元素 Ca 和 Fe 的浸出性可以忽略不计。



























 京公网安备 11010802027423号
京公网安备 11010802027423号