当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism of molecular interaction of acrylate-polyethylene glycol acrylate copolymers with calcium silicate hydrate surfaces
Green Chemistry ( IF 9.8 ) Pub Date : 2019/12/19 , DOI: 10.1039/c9gc03287h Tariq Jamil 1, 2, 3, 4 , Ali Javadi 4, 5, 6, 7 , Hendrik Heinz 1, 2, 3, 4
Green Chemistry ( IF 9.8 ) Pub Date : 2019/12/19 , DOI: 10.1039/c9gc03287h Tariq Jamil 1, 2, 3, 4 , Ali Javadi 4, 5, 6, 7 , Hendrik Heinz 1, 2, 3, 4
Affiliation
|
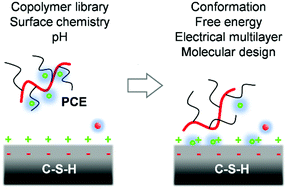
Obtaining insights into the adsorption and assembly of polyelectrolytes on chemically variable calcium silicate hydrate (C-S-H) surfaces at the atomic scale has been a longstanding challenge in the chemistry of sustainable building materials and mineral–polymer interactions. Specifically, polycarboxylate ethers (PCEs) based on acrylate and poly(ethylene glycol) acrylate co-monomers are widely used to engineer the fluidity and hydration of cement and play an important role in the search for building materials with a lower carbon footprint. We report the first systematic study of PCE interactions with C-S-H surfaces at the molecular level using simulations at single molecule coverage and comparisons to experimental data. The mechanism of adsorption of the ionic polymers is a two-step process with initial cation adsorption that reverses the mineral surface charge, followed by adsorption of the polymer backbone through ion pairing. Free energies of binding are tunable in a wide range of 0 to −5 kcal mol−1 acrylate monomer. Polymer attraction increases for higher calcium-to-silicate ratio of the mineral and higher pH value in solution, and varies significantly with PCE composition. Thereby, successive negatively charged carboxylate groups along the backbone induce conformation strain and local detachment from the surface. Polyethylene glycol (PEG) side chains in the copolymers avoid contact with the C-S-H surfaces. The results guide in the rational design of adsorption strength and conformations of the comb copolymers, and lay the groundwork to explore the vast phase space of C-S-H compositions, surface morphologies, electrolyte conditions, and PCE films of variable surface coverage. Chemically similar minerals and copolymers also find applications in other structural and biomimetic materials.
中文翻译:

丙烯酸酯-聚乙二醇丙烯酸酯共聚物与硅酸钙水合物表面的分子相互作用机理
在可持续的建筑材料和矿物-聚合物相互作用的化学过程中,获得关于聚电解质在化学可变的水合硅酸钙水合物(CSH)表面上的吸附和组装的见解一直是一项长期的挑战。具体而言,基于丙烯酸酯和聚(乙二醇)丙烯酸酯共聚单体的聚羧酸酯醚(PCE)被广泛用于设计水泥的流动性和水合作用,并在寻找具有较低碳足迹的建筑材料中发挥重要作用。我们报告了单分子覆盖率下的模拟以及与实验数据的比较,在分子水平上PCE与CSH表面相互作用的第一个系统研究。离子聚合物的吸附机理是两步过程,初始的阳离子吸附会逆转矿物表面的电荷,然后通过离子对吸附聚合物主链。结合的自由能在0到-5 kcal mol的宽范围内可调-1丙烯酸酯单体。聚合物的吸引力会随着矿物质中钙与硅酸盐的比例增加和溶液中pH值升高而增加,并且随PCE组成的变化而显着变化。因此,沿着主链的连续带负电荷的羧酸盐基团引起构象应变和从表面的局部脱离。共聚物中的聚乙二醇(PEG)侧链可避免与CSH表面接触。结果指导了梳型共聚物的吸附强度和构象的合理设计,并为探索CSH成分,表面形态,电解质条件和可变表面覆盖率的PCE膜的广阔相空间奠定了基础。化学相似的矿物和共聚物也可用于其他结构和仿生材料中。
更新日期:2020-03-09
中文翻译:

丙烯酸酯-聚乙二醇丙烯酸酯共聚物与硅酸钙水合物表面的分子相互作用机理
在可持续的建筑材料和矿物-聚合物相互作用的化学过程中,获得关于聚电解质在化学可变的水合硅酸钙水合物(CSH)表面上的吸附和组装的见解一直是一项长期的挑战。具体而言,基于丙烯酸酯和聚(乙二醇)丙烯酸酯共聚单体的聚羧酸酯醚(PCE)被广泛用于设计水泥的流动性和水合作用,并在寻找具有较低碳足迹的建筑材料中发挥重要作用。我们报告了单分子覆盖率下的模拟以及与实验数据的比较,在分子水平上PCE与CSH表面相互作用的第一个系统研究。离子聚合物的吸附机理是两步过程,初始的阳离子吸附会逆转矿物表面的电荷,然后通过离子对吸附聚合物主链。结合的自由能在0到-5 kcal mol的宽范围内可调-1丙烯酸酯单体。聚合物的吸引力会随着矿物质中钙与硅酸盐的比例增加和溶液中pH值升高而增加,并且随PCE组成的变化而显着变化。因此,沿着主链的连续带负电荷的羧酸盐基团引起构象应变和从表面的局部脱离。共聚物中的聚乙二醇(PEG)侧链可避免与CSH表面接触。结果指导了梳型共聚物的吸附强度和构象的合理设计,并为探索CSH成分,表面形态,电解质条件和可变表面覆盖率的PCE膜的广阔相空间奠定了基础。化学相似的矿物和共聚物也可用于其他结构和仿生材料中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号