当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding Loop Substitutions in the Cyclic Peptide SFTI-1 Generate Potent and Selective Chymase Inhibitors.
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.jmedchem.9b01811 Choi Yi Li 1 , Kuok Yap 1 , Joakim E Swedberg 1 , David J Craik 1 , Simon J de Veer 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.jmedchem.9b01811 Choi Yi Li 1 , Kuok Yap 1 , Joakim E Swedberg 1 , David J Craik 1 , Simon J de Veer 1
Affiliation
|
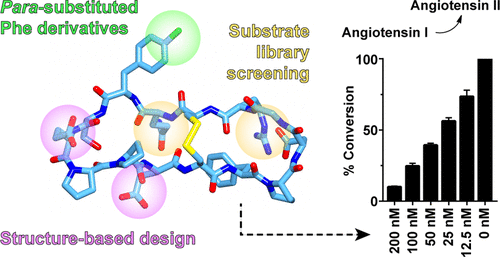
Chymase is a serine protease that is predominantly expressed by mast cells and has key roles in immune defense and the cardiovascular system. This enzyme has also emerged as a therapeutic target for cardiovascular disease due to its ability to remodel cardiac tissue and generate angiotensin II. Here, we used the nature-derived cyclic peptide sunflower trypsin inhibitor-1 (SFTI-1) as a template for designing novel chymase inhibitors. The key binding contacts of SFTI-1 were optimized by combining a peptide substrate library screen with structure-based design, which yielded several variants with potent activity. The lead variant was further modified by replacing the P1 Tyr residue with para-substituted Phe derivatives, generating new inhibitors with improved potency (Ki = 1.8 nM) and higher selectivity over closely related enzymes. Several variants were shown to block angiotensin I cleavage in vitro, highlighting their potential for further development and future evaluation as pharmaceutical leads.
中文翻译:

环肽SFTI-1中的结合环取代产生有效的选择性琼脂糖酶抑制剂。
胸苷酶是一种丝氨酸蛋白酶,主要由肥大细胞表达,在免疫防御和心血管系统中起关键作用。由于该酶具有重塑心脏组织并产生血管紧张素II的能力,因此也已成为心血管疾病的治疗靶标。在这里,我们使用自然衍生的环肽向日葵胰蛋白酶抑制剂-1(SFTI-1)作为模板来设计新型食糜酶抑制剂。通过将肽底物文库筛选与基于结构的设计相结合,可以优化SFTI-1的关键结合接触,从而产生具有强大活性的多种变体。通过用对位取代的Phe衍生物取代P1 Tyr残基,进一步修饰了先导变体,产生了新的抑制剂,这些抑制剂的效价更高(Ki = 1.8 nM),并且比紧密相关的酶具有更高的选择性。
更新日期:2020-01-09
中文翻译:

环肽SFTI-1中的结合环取代产生有效的选择性琼脂糖酶抑制剂。
胸苷酶是一种丝氨酸蛋白酶,主要由肥大细胞表达,在免疫防御和心血管系统中起关键作用。由于该酶具有重塑心脏组织并产生血管紧张素II的能力,因此也已成为心血管疾病的治疗靶标。在这里,我们使用自然衍生的环肽向日葵胰蛋白酶抑制剂-1(SFTI-1)作为模板来设计新型食糜酶抑制剂。通过将肽底物文库筛选与基于结构的设计相结合,可以优化SFTI-1的关键结合接触,从而产生具有强大活性的多种变体。通过用对位取代的Phe衍生物取代P1 Tyr残基,进一步修饰了先导变体,产生了新的抑制剂,这些抑制剂的效价更高(Ki = 1.8 nM),并且比紧密相关的酶具有更高的选择性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号