当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interactions between Soluble Species of β-Amyloid and α-Synuclein Promote Oligomerization while Inhibiting Fibrillization.
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-30 , DOI: 10.1021/acs.biochem.9b00655 Jason Candreva 1 , Edward Chau 1 , Margaret E Rice 2 , Jin Ryoun Kim 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-30 , DOI: 10.1021/acs.biochem.9b00655 Jason Candreva 1 , Edward Chau 1 , Margaret E Rice 2 , Jin Ryoun Kim 1
Affiliation
|
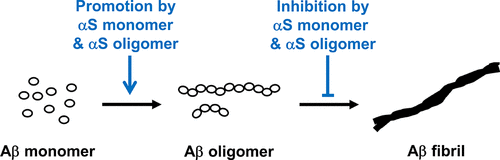
Aggregations of β-amyloid (Aβ) and α-synuclein (αS) into oligomeric and fibrillar assemblies are the pathological hallmarks of Alzheimer's and Parkinson's diseases, respectively. Although Aβ and αS affect different regions of the brain and are separated at the cellular level, there is evidence of their eventual interaction in the pathology of both disorders. Characterization of interactions of Aβ and αS at various stages of their aggregation pathways could reveal mechanisms and therapeutic targets for the prevention and cure of these neurodegenerative diseases. In this study, we comprehensively examined the interactions and their molecular manifestations using an array of characterization tools. We show for the first time that αS monomers and oligomers, but not αS fibrils, inhibit Aβ fibrillization while promoting oligomerization of Aβ monomers and stabilizing preformed Aβ oligomers via coassembly, as judged by Thioflavin T fluorescence, transmission electron microscopy, and SDS- and native-PAGE with fluorescently labeled peptides/proteins. In contrast, soluble Aβ species, such as monomers and oligomers, aggregate into fibrils, when incubated alone under the otherwise same condition. Our study provides evidence that the interactions with αS soluble species, responsible for the effects, are mediated primarily by the C-terminus of Aβ, when judged by competitive immunoassays using antibodies recognizing various fragments of Aβ. We also show that the C-terminus of Aβ is a primary site for its interaction with αS fibrils. Collectively, these data demonstrate aggregation state-specific interactions between αS and Aβ and offer insight into a molecular basis of synergistic biological effects between the two polypeptides.
中文翻译:

β-淀粉样蛋白和α-突触核蛋白的可溶性物种之间的相互作用促进了低聚反应,同时抑制了原纤维形成。
β-淀粉样蛋白(Aβ)和α-突触核蛋白(αS)聚集成寡聚体和原纤维组装体分别是阿尔茨海默氏病和帕金森氏病的病理学标志。尽管Aβ和αS影响大脑的不同区域并在细胞水平上分开,但有证据表明它们最终在两种疾病的病理学中相互作用。表征Aβ和αS在其聚集途径的各个阶段的相互作用可以揭示预防和治愈这些神经退行性疾病的机制和治疗靶标。在这项研究中,我们使用一系列表征工具全面检查了相互作用及其分子表现。我们首次展示了αS单体和低聚物,而不是αS原纤维,硫黄素T荧光,透射电镜以及SDS-和native-PAGE与荧光标记的肽/蛋白质共同判断,抑制Aβ原纤维化,同时促进Aβ单体的低聚并通过共组装稳定预先形成的Aβ低聚物。相反,当在其他相同条件下单独孵育时,可溶性Aβ物种(例如单体和低聚物)会聚集成原纤维。我们的研究提供的证据表明,当使用识别Aβ各种片段的抗体通过竞争性免疫分析法进行判断时,与影响这种作用的αS可溶性物质的相互作用主要是由Aβ的C端介导的。我们还显示,Aβ的C末端是其与αS原纤维相互作用的主要位点。总的来说,
更新日期:2019-12-30
中文翻译:

β-淀粉样蛋白和α-突触核蛋白的可溶性物种之间的相互作用促进了低聚反应,同时抑制了原纤维形成。
β-淀粉样蛋白(Aβ)和α-突触核蛋白(αS)聚集成寡聚体和原纤维组装体分别是阿尔茨海默氏病和帕金森氏病的病理学标志。尽管Aβ和αS影响大脑的不同区域并在细胞水平上分开,但有证据表明它们最终在两种疾病的病理学中相互作用。表征Aβ和αS在其聚集途径的各个阶段的相互作用可以揭示预防和治愈这些神经退行性疾病的机制和治疗靶标。在这项研究中,我们使用一系列表征工具全面检查了相互作用及其分子表现。我们首次展示了αS单体和低聚物,而不是αS原纤维,硫黄素T荧光,透射电镜以及SDS-和native-PAGE与荧光标记的肽/蛋白质共同判断,抑制Aβ原纤维化,同时促进Aβ单体的低聚并通过共组装稳定预先形成的Aβ低聚物。相反,当在其他相同条件下单独孵育时,可溶性Aβ物种(例如单体和低聚物)会聚集成原纤维。我们的研究提供的证据表明,当使用识别Aβ各种片段的抗体通过竞争性免疫分析法进行判断时,与影响这种作用的αS可溶性物质的相互作用主要是由Aβ的C端介导的。我们还显示,Aβ的C末端是其与αS原纤维相互作用的主要位点。总的来说,


























 京公网安备 11010802027423号
京公网安备 11010802027423号