Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Localized Intercellular Transfer of Ephrin-As by Trans-endocytosis Enables Long-Term Signaling.
Developmental Cell ( IF 11.8 ) Pub Date : 2019-12-13 , DOI: 10.1016/j.devcel.2019.11.013 José Ignacio Valenzuela 1 , Franck Perez 1
Developmental Cell ( IF 11.8 ) Pub Date : 2019-12-13 , DOI: 10.1016/j.devcel.2019.11.013 José Ignacio Valenzuela 1 , Franck Perez 1
Affiliation
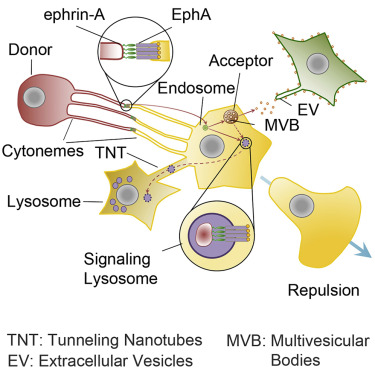
|
Ephrins can elicit either contact-mediated cell-cell adhesion or repulsion, depending on the efficiency of the removal of their ligand-receptor complexes from the cell surface, thus controlling tissue morphogenesis and oncogenic development. However, the dynamic of the turnover of newly assembled ephrin-Eph complexes during cell-cell interactions remains mostly unexplored. Here, we show that ephrin-A1-EphA2 complexes are locally formed at the tip of the filopodia, at cell-to-cell contacts. Clusters of ephrin-A1 from donor cells surf on filopodia associated to EphA2-bearing subdomains of acceptor cells. Full-length ephrin-A1 is transferred to acceptor cells by trans-endocytosis through a proteolysis-independent mechanism. Trans-endocytosed ephrin-A1 bound to its receptor enables signaling to be emitted from endo-lysosomes of acceptor cells. Localized trans-endocytosis of ephrin-A1 sustains contact-mediated repulsion on cancer cells. Our results uncover the essential role played by local concentration at the tip of filopodia and the trans-endocytosis of full-length ephrin to maintain long-lasting ephrin signaling.
中文翻译:

Ephrin-As通过跨内吞作用的局部细胞间转移使长期信号转导成为可能。
视蛋白可以引起接触介导的细胞间粘附或排斥,这取决于从细胞表面去除其配体-受体复合物的效率,从而控制组织形态发生和致癌作用的发展。然而,新组装的ephrin-Eph复合物在细胞-细胞相互作用过程中更新的动态仍然是未知的。在这里,我们显示了ephrin-A1-EphA2复合物在丝状伪足的尖端,在细胞间接触中局部形成。来自供体细胞的ephrin-A1簇在与受体细胞带有EphA2的亚结构域相关的丝状伪足上冲浪。全长ephrin-A1通过不依赖蛋白水解的机制通过内吞作用转移到受体细胞。结合到其受体的反式吞噬的ephrin-A1使信号能够从受体细胞的内溶酶体中发出。ephrin-A1的局部跨内吞作用维持了癌细胞上接触介导的排斥作用。我们的研究结果揭示了丝状伪足尖端局部浓缩和全长ephrin的内吞转运以维持持久的ephrin信号传导所起的重要作用。
更新日期:2019-12-19
中文翻译:

Ephrin-As通过跨内吞作用的局部细胞间转移使长期信号转导成为可能。
视蛋白可以引起接触介导的细胞间粘附或排斥,这取决于从细胞表面去除其配体-受体复合物的效率,从而控制组织形态发生和致癌作用的发展。然而,新组装的ephrin-Eph复合物在细胞-细胞相互作用过程中更新的动态仍然是未知的。在这里,我们显示了ephrin-A1-EphA2复合物在丝状伪足的尖端,在细胞间接触中局部形成。来自供体细胞的ephrin-A1簇在与受体细胞带有EphA2的亚结构域相关的丝状伪足上冲浪。全长ephrin-A1通过不依赖蛋白水解的机制通过内吞作用转移到受体细胞。结合到其受体的反式吞噬的ephrin-A1使信号能够从受体细胞的内溶酶体中发出。ephrin-A1的局部跨内吞作用维持了癌细胞上接触介导的排斥作用。我们的研究结果揭示了丝状伪足尖端局部浓缩和全长ephrin的内吞转运以维持持久的ephrin信号传导所起的重要作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号