当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo.
Journal of Controlled Release ( IF 10.8 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.jconrel.2019.12.032 Petro Zhupanyn 1 , Alexander Ewe 1 , Thomas Büch 1 , Anastasia Malek 2 , Phil Rademacher 3 , Claudia Müller 4 , Anja Reinert 5 , Yarúa Jaimes 3 , Achim Aigner 1
Journal of Controlled Release ( IF 10.8 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.jconrel.2019.12.032 Petro Zhupanyn 1 , Alexander Ewe 1 , Thomas Büch 1 , Anastasia Malek 2 , Phil Rademacher 3 , Claudia Müller 4 , Anja Reinert 5 , Yarúa Jaimes 3 , Achim Aigner 1
Affiliation
|
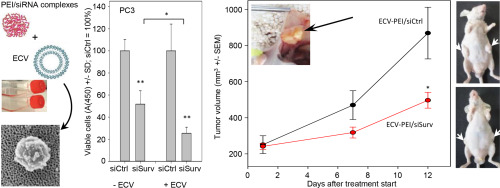
Extracellular vesicles (ECVs) are secreted cell-derived membrane particles involved in intercellular signaling and cell-cell communication. By transporting various bio-macromolecules, ECVs and in particular exosomes are relevant in various (patho-) physiological processes. ECVs are also released by cancer cells and can confer pro-tumorigenic effects. Their target cell tropism, effects on proliferation rates, natural stability in blood and immunotolerance makes ECVs particularly interesting as delivery vehicles. Polyethylenimines (PEIs) are linear or branched polymers which are capable of forming non-covalent complexes with small RNA molecules including siRNAs or antimiRs, for their delivery in vitro and in vivo. This study explores for the first time the combination of PEI-based nanoparticles with naturally occurring ECVs from different cell lines, for the delivery of small RNAs. ECV-modified PEI/siRNA complexes are analyzed by electron microscopy vs. ECV or complex alone. On the functional side, we demonstrate increased knockdown efficacy and storage stability of PEI/siRNA complexes upon their modification with ECVs. This is paralleled by enhanced tumor cell-inhibition by ECV-modified PEI/siRNA complexes targeting Survivin. Pre-treatment with various inhibitors of cellular internalization reveals alterations in cellular uptake mechanisms and biological activities of PEI/siRNA complexes upon their ECV modification. Extending our studies towards PEI-complexed antimiRs against miR-155 or miR-1246, dose-dependent cellular and molecular effects are enhanced in ECV-modified complexes, based on the de-repression of direct miRNA target genes. Differences between ECVs from different cell lines are observed regarding their capacity of enhancing PEI/siRNA efficacies, independent of the target cell line for transfection. Finally, an in vivo therapy study in mice bearing s.c. PC3 prostate carcinoma xenografts reveals marked inhibition of tumor growth upon treatment with ECVPC3-modified PEI/siSurvivin complexes, based on profound target gene knockdown. We conclude that ECV-modification enhances the activity of PEI-based complexes, by altering pivotal physicochemical and biological nanoparticle properties.
中文翻译:

细胞外囊泡(ECV)修饰的聚乙烯亚胺(PEI)复合物可增强体内和体外的siRNA递送。
细胞外囊泡(ECV)是分泌的细胞衍生膜颗粒,参与细胞间信号传导和细胞间通讯。通过运输各种生物大分子,ECV,尤其是外来体与各种(病理)生理过程有关。ECV也可被癌细胞释放,并可赋予促癌作用。它们的靶细胞嗜性,对增殖率的影响,血液中的自然稳定性和免疫耐受性使ECV作为输送工具尤为有趣。聚乙烯亚胺(PEI)是线型或支链聚合物,能够与包括siRNA或antimiR在内的小RNA分子形成非共价复合物,以在体内和体外递送。这项研究首次探索了基于PEI的纳米粒子与来自不同细胞系的天然ECV的结合,用于小RNA的递送。ECV修饰的PEI / siRNA复合物通过电子显微镜与ECV或单独复合物进行分析。在功能方面,我们证明了用ECV修饰PEI / siRNA复合物后,其敲除功效和储存稳定性均得到提高。这与靶向Survivin的ECV修饰的PEI / siRNA复合物增强的肿瘤细胞抑制作用平行。用各种细胞内在化抑制剂进行的预处理显示,PEI / siRNA复合物的ECV修饰后,其细胞摄取机制和生物学活性会发生变化。将我们的研究扩展到针对miR-155或miR-1246的PEI复合抗miR,基于直接miRNA靶基因的抑制,ECV修饰的复合物中剂量依赖性细胞和分子效应得到增强。观察到来自不同细胞系的ECV之间在增强PEI / siRNA效力方面的差异,而与目标细胞系的转染无关。最后,一项基于sc PC3前列腺癌异种移植物的小鼠体内治疗研究显示,基于深刻的靶基因敲低,用ECVPC3修饰的PEI / siSurvivin复合物治疗后,肿瘤生长受到了明显的抑制。我们得出的结论是,ECV修饰通过改变关键的理化和生物纳米粒子特性来增强基于PEI的复合物的活性。基于深刻的靶基因敲低,PC3前列腺癌异种移植物显着抑制了ECVPC3修饰的PEI / siSurvivin复合物治疗后肿瘤的生长。我们得出的结论是,ECV修饰通过改变关键的理化和生物纳米粒子特性来增强基于PEI的复合物的活性。基于深刻的靶基因敲低,PC3前列腺癌异种移植物在用ECVPC3修饰的PEI / siSurvivin复合物治疗后显示出对肿瘤生长的显着抑制。我们得出的结论是,ECV修饰通过改变关键的理化和生物纳米粒子特性来增强基于PEI的复合物的活性。
更新日期:2019-12-19
中文翻译:

细胞外囊泡(ECV)修饰的聚乙烯亚胺(PEI)复合物可增强体内和体外的siRNA递送。
细胞外囊泡(ECV)是分泌的细胞衍生膜颗粒,参与细胞间信号传导和细胞间通讯。通过运输各种生物大分子,ECV,尤其是外来体与各种(病理)生理过程有关。ECV也可被癌细胞释放,并可赋予促癌作用。它们的靶细胞嗜性,对增殖率的影响,血液中的自然稳定性和免疫耐受性使ECV作为输送工具尤为有趣。聚乙烯亚胺(PEI)是线型或支链聚合物,能够与包括siRNA或antimiR在内的小RNA分子形成非共价复合物,以在体内和体外递送。这项研究首次探索了基于PEI的纳米粒子与来自不同细胞系的天然ECV的结合,用于小RNA的递送。ECV修饰的PEI / siRNA复合物通过电子显微镜与ECV或单独复合物进行分析。在功能方面,我们证明了用ECV修饰PEI / siRNA复合物后,其敲除功效和储存稳定性均得到提高。这与靶向Survivin的ECV修饰的PEI / siRNA复合物增强的肿瘤细胞抑制作用平行。用各种细胞内在化抑制剂进行的预处理显示,PEI / siRNA复合物的ECV修饰后,其细胞摄取机制和生物学活性会发生变化。将我们的研究扩展到针对miR-155或miR-1246的PEI复合抗miR,基于直接miRNA靶基因的抑制,ECV修饰的复合物中剂量依赖性细胞和分子效应得到增强。观察到来自不同细胞系的ECV之间在增强PEI / siRNA效力方面的差异,而与目标细胞系的转染无关。最后,一项基于sc PC3前列腺癌异种移植物的小鼠体内治疗研究显示,基于深刻的靶基因敲低,用ECVPC3修饰的PEI / siSurvivin复合物治疗后,肿瘤生长受到了明显的抑制。我们得出的结论是,ECV修饰通过改变关键的理化和生物纳米粒子特性来增强基于PEI的复合物的活性。基于深刻的靶基因敲低,PC3前列腺癌异种移植物显着抑制了ECVPC3修饰的PEI / siSurvivin复合物治疗后肿瘤的生长。我们得出的结论是,ECV修饰通过改变关键的理化和生物纳米粒子特性来增强基于PEI的复合物的活性。基于深刻的靶基因敲低,PC3前列腺癌异种移植物在用ECVPC3修饰的PEI / siSurvivin复合物治疗后显示出对肿瘤生长的显着抑制。我们得出的结论是,ECV修饰通过改变关键的理化和生物纳米粒子特性来增强基于PEI的复合物的活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号