当前位置:
X-MOL 学术
›
J. Pharmaceut. Biomed. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An enantiomeric quantitative LC-MS/MS method for tolvaptan and its monohydroxylates in human plasma using a reversed-phase separation procedure.
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.4 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.jpba.2019.113061 Shunta Akutsu 1 , Takafumi Naito 1 , Kohei Hoshikawa 1 , Masao Saotome 2 , Yuichiro Maekawa 2 , Junichi Kawakami 1
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.4 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.jpba.2019.113061 Shunta Akutsu 1 , Takafumi Naito 1 , Kohei Hoshikawa 1 , Masao Saotome 2 , Yuichiro Maekawa 2 , Junichi Kawakami 1
Affiliation
|
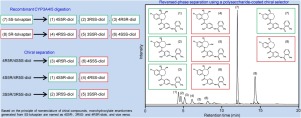
Racemic tolvaptan possessing an asymmetric carbon is metabolized to three pairs of monohydroxylate enantiomers of diol form with V2 receptor antagonistic activity via CYP3A. This study aimed to develop a simultaneous quantitative liquid chromatography-tandem mass spectrometry method for 5R- and 5S-tolvaptan and their monohydroxylate enantiomers in human plasma and to apply it to patient samples. Deproteinized plasma specimens were separated using a polysaccharide derivative chiral column in a reversed-phase elution mode. The mass spectrometer was run in positive ion electrospray ionization mode. The chromatographic peaks of tolvaptan monohydroxylate enantiomers were identified by the recombinant CYP3A4/5 digestion of 5R- and 5S-tolvaptan. The calibration curves ranged over the plasma concentrations of 0.25-125 ng/mL for 5R- and 5S-tolvaptan, 0.025-12.5 ng/mL for 4R5R- and 4S5S-diols, and 0.025-38.15 ng/mL for 4S5R-, 4R5S-, 3S5R-, and 3R5S-diols with a large variation. Their pre-treatment recovery rates and matrix factors in human plasma were 85.2-112.9 % and 86.9-113.1 %, respectively. The intra- and inter-day accuracy and imprecision were 92.3-113.8 % and 3.5-14.6 % for all analytes, respectively. The plasma concentration ranges of 5R- and 5S-tolvaptan, 4R5R-, 4S5S-, 4S5R-, 4R5S-, 3S5R-, and 3R5S-diols in heart failure patients with a 5-fold dilution procedure were 0.634-28.4, 2.37-131, 0.525-15.4, 0.0970-4.08, 6.82-108, 0.271-6.49, 0.394-4.18, and 4.81-39.8 ng/mL, respectively. In conclusion, the present method has an acceptable analytical performance level and can be helpful for characterization of the plasma 5R- and 5S-tolvaptan and their monohydroxylate enantiomers in heart failure patients.
中文翻译:

使用反相分离程序测定人血浆中托伐普坦及其单羟基化物的对映体定量LC-MS / MS方法。
具有不对称碳的外消旋托伐普坦通过CYP3A代谢为三对具有V2受体拮抗活性的二醇形式的单羟基化对映体。这项研究的目的是开发一种同时定量液相色谱-串联质谱法测定人血浆中5R-和5S-托伐普坦及其单羟基化对映体的方法,并将其应用于患者样品。用多糖衍生物手性柱以反相洗脱模式分离去蛋白血浆样品。质谱仪以正离子电喷雾电离模式运行。通过5R-和5S-托伐普坦的重组CYP3A4 / 5消化,鉴定了托伐普坦单羟基化对映体的色谱峰。对于5R-和5S-托伐普坦,0的血浆浓度在0.25-125 ng / mL的血浆浓度范围内校准曲线。对于4R5R-和4S5S-二醇为025-12.5 ng / mL,对于4S5R-,4R5S-,3S5R-和3R5S-二醇变化较大的为0.025-38.15 ng / mL。它们在人血浆中的预处理回收率和基质因子分别为85.2-112.9%和86.9-113.1%。所有分析物的日内和日间准确性和不准确性分别为92.3-113.8%和3.5-14.6%。使用5倍稀释程序的心力衰竭患者中5R-和5S-托伐普坦,4R5R-,4S5S-,4S5R-,4R5S-,3S5R-和3R5S-二醇的血浆浓度范围为0.634-28.4、2.37-131分别为0.525-15.4、0.0970-4.08、6.82-108、0.271-6.49、0.394-4.18和4.81-39.8 ng / mL。综上所述,
更新日期:2019-12-19
中文翻译:

使用反相分离程序测定人血浆中托伐普坦及其单羟基化物的对映体定量LC-MS / MS方法。
具有不对称碳的外消旋托伐普坦通过CYP3A代谢为三对具有V2受体拮抗活性的二醇形式的单羟基化对映体。这项研究的目的是开发一种同时定量液相色谱-串联质谱法测定人血浆中5R-和5S-托伐普坦及其单羟基化对映体的方法,并将其应用于患者样品。用多糖衍生物手性柱以反相洗脱模式分离去蛋白血浆样品。质谱仪以正离子电喷雾电离模式运行。通过5R-和5S-托伐普坦的重组CYP3A4 / 5消化,鉴定了托伐普坦单羟基化对映体的色谱峰。对于5R-和5S-托伐普坦,0的血浆浓度在0.25-125 ng / mL的血浆浓度范围内校准曲线。对于4R5R-和4S5S-二醇为025-12.5 ng / mL,对于4S5R-,4R5S-,3S5R-和3R5S-二醇变化较大的为0.025-38.15 ng / mL。它们在人血浆中的预处理回收率和基质因子分别为85.2-112.9%和86.9-113.1%。所有分析物的日内和日间准确性和不准确性分别为92.3-113.8%和3.5-14.6%。使用5倍稀释程序的心力衰竭患者中5R-和5S-托伐普坦,4R5R-,4S5S-,4S5R-,4R5S-,3S5R-和3R5S-二醇的血浆浓度范围为0.634-28.4、2.37-131分别为0.525-15.4、0.0970-4.08、6.82-108、0.271-6.49、0.394-4.18和4.81-39.8 ng / mL。综上所述,



























 京公网安备 11010802027423号
京公网安备 11010802027423号