当前位置:
X-MOL 学术
›
Corros. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Further insights into the mechanisms involved in the corrosion of 316L(N) austenitic steel in oxygenated liquid sodium at 550 °C
Corrosion Science ( IF 8.3 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.corsci.2019.108399 Matthieu Rivollier , Jean-Louis Courouau , Michel Tabarant , Cécile Blanc , François Jomard , Marie-Laurence Giorgi
Corrosion Science ( IF 8.3 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.corsci.2019.108399 Matthieu Rivollier , Jean-Louis Courouau , Michel Tabarant , Cécile Blanc , François Jomard , Marie-Laurence Giorgi
|
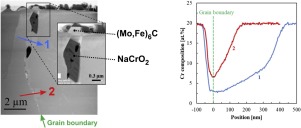
Abstract 316 L(N) austenitic steel was corroded at 550 °C in liquid sodium containing 200 μg g−1 dissolved oxygen from 242 h to 7704 h. NaCrO2 is formed for all immersion times, O and Na from liquid metal and Cr from steel. The oxidation front is located at the chromite / steel interface and in the steel grain boundaries. M6C carbides (M = Mo and Fe) are also formed, C from liquid sodium. NaCrO2 is dissolved until the saturation of liquid sodium in Cr is reached. The Cr solubility is higher than that of pure Cr due to the presence of dissolved oxygen.
中文翻译:

进一步了解 316L(N) 奥氏体钢在 550 °C 含氧液态钠中的腐蚀机制
摘要 316 L(N) 奥氏体钢在 550 °C 下在含有 200 μg g-1 溶解氧的液态钠中腐蚀 242 小时至 7704 小时。NaCrO2 在所有浸泡时间中都会形成,O 和 Na 来自液态金属,Cr 来自钢。氧化前沿位于铬铁矿/钢界面和钢晶界处。还形成 M6C 碳化物(M = Mo 和 Fe),C 来自液态钠。NaCrO2 被溶解,直到达到液态钠在 Cr 中的饱和度。由于溶解氧的存在,Cr 的溶解度高于纯 Cr 的溶解度。
更新日期:2020-04-01
中文翻译:

进一步了解 316L(N) 奥氏体钢在 550 °C 含氧液态钠中的腐蚀机制
摘要 316 L(N) 奥氏体钢在 550 °C 下在含有 200 μg g-1 溶解氧的液态钠中腐蚀 242 小时至 7704 小时。NaCrO2 在所有浸泡时间中都会形成,O 和 Na 来自液态金属,Cr 来自钢。氧化前沿位于铬铁矿/钢界面和钢晶界处。还形成 M6C 碳化物(M = Mo 和 Fe),C 来自液态钠。NaCrO2 被溶解,直到达到液态钠在 Cr 中的饱和度。由于溶解氧的存在,Cr 的溶解度高于纯 Cr 的溶解度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号