当前位置:
X-MOL 学术
›
J. Mol. Cell. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dissection of heterocellular cross-talk in vascularized cardiac tissue mimetics.
Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.yjmcc.2019.12.005 Julian Uwe Gabriel Wagner 1 , Minh Duc Pham 2 , Luka Nicin 3 , Marie Hammer 3 , Katharina Bottermann 3 , Ting Yuan 3 , Rahul Sharma 2 , David John 3 , Marion Muhly-Reinholz 3 , Lukas Tombor 3 , Martin Hardt 4 , Josef Madl 5 , Stefanie Dimmeler 6 , Jaya Krishnan 7
Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.yjmcc.2019.12.005 Julian Uwe Gabriel Wagner 1 , Minh Duc Pham 2 , Luka Nicin 3 , Marie Hammer 3 , Katharina Bottermann 3 , Ting Yuan 3 , Rahul Sharma 2 , David John 3 , Marion Muhly-Reinholz 3 , Lukas Tombor 3 , Martin Hardt 4 , Josef Madl 5 , Stefanie Dimmeler 6 , Jaya Krishnan 7
Affiliation
|
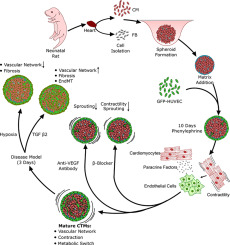
Cellular specialization and interaction with other cell types in cardiac tissue is essential for the coordinated function of cell populations in the heart. The complex interplay between cardiomyocytes, endothelial cells and fibroblasts is necessary for adaptation but can also lead to pathophysiological remodeling. To understand this complex interplay, we developed 3D vascularized cardiac tissue mimetics (CTM) to study heterocellular cross-talk in hypertrophic, hypoxic and fibrogenic environments. This 3D platform responds to physiologic and pathologic stressors and mimics the microenvironment of diseased tissue. In combination with endothelial cell fluorescence reporters, these cardiac tissue mimetics can be used to precisely visualize and quantify cellular and functional responses upon stress stimulation. Utilizing this platform, we demonstrate that stimulation of α/β-adrenergic receptors with phenylephrine (PE) promotes cardiomyocyte hypertrophy, metabolic maturation and vascularization of CTMs. Increased vascularization was promoted by conditioned medium of PE-stimulated cardiomyocytes and blocked by inhibiting VEGF or upon β-adrenergic receptor antagonist treatment, demonstrating cardiomyocyte-endothelial cross-talk. Pathophysiological stressors such as severe hypoxia reduced angiogenic sprouting and increased cell death, while TGF β2 stimulation increased collagen deposition concomitant to endothelial-to-mesenchymal transition. In sum, we have developed a cardiac 3D culture system that reflects native cardiac tissue function, metabolism and morphology - and for the first time enables the tracking and analysis of cardiac vascularization dynamics in physiology and pathology.
中文翻译:

解剖血管化心脏组织模拟物中的异细胞串扰。
心脏组织中的细胞专业化以及与其他细胞类型的相互作用对于心脏中细胞群的协调功能至关重要。心肌细胞,内皮细胞和成纤维细胞之间复杂的相互作用是适应所必需的,但也可能导致病理生理重塑。为了了解这种复杂的相互作用,我们开发了3D血管化心脏组织模拟物(CTM),以研究肥大,低氧和纤维化环境中的异细胞串扰。该3D平台可响应生理和病理应激源,并模拟患病组织的微环境。与内皮细胞荧光报告物结合使用时,这些心脏组织模拟物可用于精确可视化和量化应激刺激后的细胞和功能反应。利用这个平台,我们证明用去氧肾上腺素(PE)刺激α/β-肾上腺素能受体可促进心肌细胞肥大,代谢成熟和CTM的血管形成。PE刺激的心肌细胞的条件培养基促进血管形成的增加,并通过抑制VEGF或经β-肾上腺素受体拮抗剂治疗而被阻断,证明了心肌细胞与内皮的串扰。病理生理应激源(例如严重的缺氧)减少了血管新生,并增加了细胞死亡,而TGFβ2刺激增加了胶原蛋白的沉积,伴随着内皮向间充质的转化。总而言之,我们开发了一种可反映天然心脏组织功能的心脏3D培养系统,
更新日期:2019-12-19
中文翻译:

解剖血管化心脏组织模拟物中的异细胞串扰。
心脏组织中的细胞专业化以及与其他细胞类型的相互作用对于心脏中细胞群的协调功能至关重要。心肌细胞,内皮细胞和成纤维细胞之间复杂的相互作用是适应所必需的,但也可能导致病理生理重塑。为了了解这种复杂的相互作用,我们开发了3D血管化心脏组织模拟物(CTM),以研究肥大,低氧和纤维化环境中的异细胞串扰。该3D平台可响应生理和病理应激源,并模拟患病组织的微环境。与内皮细胞荧光报告物结合使用时,这些心脏组织模拟物可用于精确可视化和量化应激刺激后的细胞和功能反应。利用这个平台,我们证明用去氧肾上腺素(PE)刺激α/β-肾上腺素能受体可促进心肌细胞肥大,代谢成熟和CTM的血管形成。PE刺激的心肌细胞的条件培养基促进血管形成的增加,并通过抑制VEGF或经β-肾上腺素受体拮抗剂治疗而被阻断,证明了心肌细胞与内皮的串扰。病理生理应激源(例如严重的缺氧)减少了血管新生,并增加了细胞死亡,而TGFβ2刺激增加了胶原蛋白的沉积,伴随着内皮向间充质的转化。总而言之,我们开发了一种可反映天然心脏组织功能的心脏3D培养系统,


























 京公网安备 11010802027423号
京公网安备 11010802027423号