当前位置:
X-MOL 学术
›
Phytomedicine
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
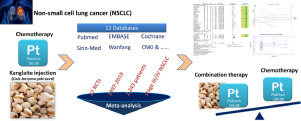
Kanglaite injection plus platinum-based chemotherapy for stage III/IV non-small cell lung cancer: A meta-analysis of 27 RCTs.
Phytomedicine ( IF 7.9 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.phymed.2019.153154 Xiaoming Huang 1 , Jue Wang 1 , Wanjun Lin 1 , Na Zhang 1 , Jingjing Du 1 , Ze Long 1 , You Yang 1 , Bowen Zheng 1 , Fangfang Zhong 1 , Qibiao Wu 1 , Wenzhe Ma 1
Phytomedicine ( IF 7.9 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.phymed.2019.153154 Xiaoming Huang 1 , Jue Wang 1 , Wanjun Lin 1 , Na Zhang 1 , Jingjing Du 1 , Ze Long 1 , You Yang 1 , Bowen Zheng 1 , Fangfang Zhong 1 , Qibiao Wu 1 , Wenzhe Ma 1
Affiliation
|
BACKGROUND
Kanglaite injection (KLT) is a broad-spectrum anti-tumor drug, which is extracted from the seeds of the Chinese medicinal herb Coix lacryma-jobi, and has been widely used for the treatment of advanced lung cancer.
PURPOSE
To evaluate the combined effects of Kanglaite injection plus platinum-based chemotherapy (PBC) on patients with stage III/IV non-small cell lung cancer (NSCLC).
STUDY DESIGN
A systematic review and meta-analysis of randomized clinical trials (RCTs).
MATERIALS AND METHODS
Twelve databases were searched from their inceptions until July 05, 2019. All the RCTs comparing the efficacy and safety of Kanglaite injection plus PBC versus PBC alone were selected. Analyses were performed using Review Manager 5.3, Comprehensive Meta-Analysis 3.0 and Trial Sequential Analysis (TSA). Disease control rate (DCR) was defined as the primary endpoint, objective response rate (ORR), survival rate, quality of life (QOL), cellular immunity function, and toxicities were defined as the secondary endpoints.
RESULTS
Twenty-seven RCTs recruiting 2,243 patients with stage III/IV NSCLC were included. The results showed that, compared with PBC alone, Kanglaite injection plus PBC improved DCR (RR = 1.20, 95% CI 1.15-1.26, p < 0.00001), ORR (RR = 1.45, 95% CI 1.31-1.60, p < 0.00001), 1-year survival rate (RR = 1.20, 95% CI 1.02-1.43, p = 0.03), QOL (RR = 1.32, 95% CI 1.25-1.40, p < 0.00001), CD4+T cells (WMD = 4.86, 95% CI 4.00-5.73, p < 0.00001), CD4+/CD8+ ratio (WMD = 0.19, 95% CI 0.07-0.31, p < 0.002), and reduced severe toxicities by 59% (RR = 0.41, 95% CI 0.33-0.51, p < 0.00001). Most results were robust and the quality of evidence was from moderate to low.
CONCLUSIONS
Kanglaite injection in combination with PBC showed significantly higher efficacy than PBC alone in the treatment of stage III/IV NSCLC. Moreover, the combination therapy can improve cellular immunity and attenuate the severe toxicities caused by chemotherapy. However, high-quality RCTs are warranted to further assess the effects of the combined therapy.
中文翻译:

康莱特注射液加铂类化学疗法治疗III / IV期非小细胞肺癌:对27个RCT的荟萃分析。
背景技术康莱特注射液(KLT)是一种广谱抗肿瘤药物,是从中草药Co仁种子中提取的,已广泛用于晚期肺癌的治疗。目的评估康莱特注射液联合铂类化学疗法(PBC)对III / IV期非小细胞肺癌(NSCLC)患者的综合作用。研究设计对随机临床试验(RCT)进行系统回顾和荟萃分析。材料与方法从开始至2019年7月5日,共检索了十二个数据库。选择了所有比较康莱特注射液加PBC与单独PBC的疗效和安全性的RCT。使用Review Manager 5.3,Comprehensive Meta-Analysis 3.0和试验顺序分析(TSA)进行分析。疾病控制率(DCR)被定义为主要终点,客观反应率(ORR),存活率,生活质量(QOL),细胞免疫功能和毒性被定义为主要终点。结果纳入了27项RCT,招募了2,243例III / IV期NSCLC患者。结果表明,与单独的PBC相比,康莱特注射液加PBC改善了DCR(RR = 1.20,95%CI 1.15-1.26,p <0.00001),ORR(RR = 1.45,95%CI 1.31-1.60,p <0.00001) ,1年生存率(RR = 1.20,95%CI 1.02-1.43,p = 0.03),QOL(RR = 1.32,95%CI 1.25-1.40,p <0.00001),CD4 + T细胞(WMD = 4.86, 95%CI 4.00-5.73,p <0.00001),CD4 + / CD8 +比(WMD = 0.19,95%CI 0.07-0.31,p <0.002),严重毒性降低了59%(RR = 0.41,95%CI 0.33-) 0.51,p <0.00001)。大多数结果是可靠的,证据的质量是中度到低度。结论康莱特注射液与PBC联合治疗III / IV期NSCLC的疗效显着高于单独的PBC。而且,联合疗法可以提高细胞免疫力并减轻由化学疗法引起的严重毒性。但是,需要高质量的RCT来进一步评估联合治疗的效果。
更新日期:2019-12-19
中文翻译:

康莱特注射液加铂类化学疗法治疗III / IV期非小细胞肺癌:对27个RCT的荟萃分析。
背景技术康莱特注射液(KLT)是一种广谱抗肿瘤药物,是从中草药Co仁种子中提取的,已广泛用于晚期肺癌的治疗。目的评估康莱特注射液联合铂类化学疗法(PBC)对III / IV期非小细胞肺癌(NSCLC)患者的综合作用。研究设计对随机临床试验(RCT)进行系统回顾和荟萃分析。材料与方法从开始至2019年7月5日,共检索了十二个数据库。选择了所有比较康莱特注射液加PBC与单独PBC的疗效和安全性的RCT。使用Review Manager 5.3,Comprehensive Meta-Analysis 3.0和试验顺序分析(TSA)进行分析。疾病控制率(DCR)被定义为主要终点,客观反应率(ORR),存活率,生活质量(QOL),细胞免疫功能和毒性被定义为主要终点。结果纳入了27项RCT,招募了2,243例III / IV期NSCLC患者。结果表明,与单独的PBC相比,康莱特注射液加PBC改善了DCR(RR = 1.20,95%CI 1.15-1.26,p <0.00001),ORR(RR = 1.45,95%CI 1.31-1.60,p <0.00001) ,1年生存率(RR = 1.20,95%CI 1.02-1.43,p = 0.03),QOL(RR = 1.32,95%CI 1.25-1.40,p <0.00001),CD4 + T细胞(WMD = 4.86, 95%CI 4.00-5.73,p <0.00001),CD4 + / CD8 +比(WMD = 0.19,95%CI 0.07-0.31,p <0.002),严重毒性降低了59%(RR = 0.41,95%CI 0.33-) 0.51,p <0.00001)。大多数结果是可靠的,证据的质量是中度到低度。结论康莱特注射液与PBC联合治疗III / IV期NSCLC的疗效显着高于单独的PBC。而且,联合疗法可以提高细胞免疫力并减轻由化学疗法引起的严重毒性。但是,需要高质量的RCT来进一步评估联合治疗的效果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号