当前位置:
X-MOL 学术
›
Phytomedicine
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
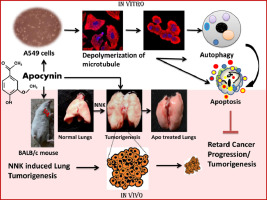
Targeting cellular microtubule by phytochemical apocynin exhibits autophagy-mediated apoptosis to inhibit lung carcinoma progression and tumorigenesis.
Phytomedicine ( IF 7.9 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.phymed.2019.153152 Santanu Paul 1 , Subhendu Chakrabarty 2 , Suvranil Ghosh 3 , Debasish Nag 1 , Amlan Das 4 , Debabrata Ghosh Dastidar 5 , Moumita Dasgupta 1 , Naibedya Dutta 3 , Mandavi Kumari 1 , Mahadeb Pal 3 , Gopal Chakrabarti 1
Phytomedicine ( IF 7.9 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.phymed.2019.153152 Santanu Paul 1 , Subhendu Chakrabarty 2 , Suvranil Ghosh 3 , Debasish Nag 1 , Amlan Das 4 , Debabrata Ghosh Dastidar 5 , Moumita Dasgupta 1 , Naibedya Dutta 3 , Mandavi Kumari 1 , Mahadeb Pal 3 , Gopal Chakrabarti 1
Affiliation
|
BACKGROUND
Lung cancer is the leading cause of cancer-related deaths worldwide. Several targets have been identified for lung cancer therapy, amongst which 'Microtubule' and its dynamics are the most widely studied and used in therapy. Tubulin-microtubule polymer dynamics are highly sought after targets in the field of anti-cancer drug designing. Natural compounds are important sources for developing anticancer therapeutics owing to their efficacy and lower cytotoxicity. Evidence suggested that therapeutic targeting of microtubule by natural compounds is amongst the most widely used interventions in numerous cancer therapies including lung cancer.
PURPOSE
To determine the efficacy of apocynin (a natural compound) in suppressing the progression of lung carcinoma both in vitro and in vivo, along with the identification of targets and the underlying mechanism for developing a novel therapeutic approach.
METHODS
We have demonstrated themicrotubule depolymerizing role of apocynin by established protocols in cellular and cell-free system. The efficacy of apocynin to inhibit lung carcinoma progression was studied on A549 cells.The tumoricidal ability of apocynin was studied in BALB/c mice model as well.Mice were classified into 4 groups namely-group II mice as tumor control; group III-IV mice asalso tumor-induced but treated with differential apocynin doses whereas group I mice were kept as normal.
RESULTS
Apocynin, showed selective cytotoxicity towards lung cancer cells rather than normal lung fibroblast cells. Apocynin inhibited oncogenic properties including growth, proliferation (p < 0.05), colony formation (p < 0.05), invasion (p < 0.05) and spheroid formation (p < 0.05) in lung cancer cells. Apart from other established properties, apocynin was found to be a novel and potent component to bind with tubulin and depolymerize cellular microtubule network. Apocynin mediated cellular microtubule depolymerization was the driving mechanism to trigger autophagy-mediated apoptotic cell death (p < 0.05) which in turn retarded lung cancer progression. Furthermore, apocynin showed tumoricidal characteristics to inhibit lung tumorigenesis in mice as well.
CONCLUSION
Targeting tubulin-microtubule equilibrium with apocynin could be the key regulator to catastrophe cellular catabolic processes to mitigate lung carcinoma. Thus, apocynin could be a potential therapeutic agent for lung cancer treatment.
中文翻译:

通过植物化学载脂蛋白的靶向细胞微管表现出自噬介导的凋亡,以抑制肺癌的进展和肿瘤发生。
背景技术肺癌是世界范围内与癌症相关的死亡的主要原因。已经确定了用于肺癌治疗的几个靶标,其中“微管”及其动力学被最广泛地研究并用于治疗。微管蛋白-微管聚合物动力学是抗癌药物设计领域的目标。由于天然化合物的功效和较低的细胞毒性,它们是开发抗癌治疗剂的重要来源。有证据表明,天然化合物对微管的靶向治疗是包括肺癌在内的许多癌症治疗方法中使用最广泛的干预措施之一。目的确定载脂蛋白(一种天然化合物)在体外和体内抑制肺癌进展的功效,以及目标的确定和开发新型治疗方法的潜在机制。方法我们已经通过在细胞和无细胞系统中建立的方案证明了载脂蛋白的微管解聚作用。研究了Apocynin对肺癌细胞A549的抑制作用。还研究了Apocynin在BALB / c小鼠模型中的抑瘤能力。将小鼠分为4组,即II组作为对照组。III-IV组小鼠也被肿瘤诱导,但是用不同剂量的阿朴西宁剂量处理,而I组小鼠保持正常。结果Apocynin对肺癌细胞而非正常肺成纤维细胞具有选择性的细胞毒性。Apocynin抑制致癌特性,包括生长,增殖(p <0.05),菌落形成(p <0.05),肺癌细胞的浸润(p <0.05)和球状形成(p <0.05)。除其他已确立的特性外,载脂蛋白还被发现是一种新颖且有效的成分,可与微管蛋白结合并解聚细胞微管网络。Apocynin介导的细胞微管解聚是触发自噬介导的凋亡细胞死亡(p <0.05)的驱动机制,继而延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。05)在肺癌细胞中。除其他已确立的特性外,载脂蛋白还被发现是一种新颖且有效的成分,可与微管蛋白结合并解聚细胞微管网络。Apocynin介导的细胞微管解聚是触发自噬介导的凋亡细胞死亡(p <0.05)的驱动机制,继而延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。05)在肺癌细胞中。除其他已确立的特性外,载脂蛋白还被发现是一种新颖且有效的成分,可与微管蛋白结合并解聚细胞微管网络。Apocynin介导的细胞微管解聚是触发自噬介导的凋亡细胞死亡(p <0.05)的驱动机制,继而延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。发现载脂蛋白是与微管蛋白结合并解聚细胞微管网络的新型有效成分。Apocynin介导的细胞微管解聚是触发自噬介导的凋亡细胞死亡(p <0.05)的驱动机制,继而延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。发现载脂蛋白是与微管蛋白结合并解聚细胞微管网络的新型有效成分。Apocynin介导的细胞微管解聚是触发自噬介导的凋亡细胞死亡(p <0.05)的驱动机制,继而延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。05)反过来又延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。05)反过来又延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。
更新日期:2019-12-19
中文翻译:

通过植物化学载脂蛋白的靶向细胞微管表现出自噬介导的凋亡,以抑制肺癌的进展和肿瘤发生。
背景技术肺癌是世界范围内与癌症相关的死亡的主要原因。已经确定了用于肺癌治疗的几个靶标,其中“微管”及其动力学被最广泛地研究并用于治疗。微管蛋白-微管聚合物动力学是抗癌药物设计领域的目标。由于天然化合物的功效和较低的细胞毒性,它们是开发抗癌治疗剂的重要来源。有证据表明,天然化合物对微管的靶向治疗是包括肺癌在内的许多癌症治疗方法中使用最广泛的干预措施之一。目的确定载脂蛋白(一种天然化合物)在体外和体内抑制肺癌进展的功效,以及目标的确定和开发新型治疗方法的潜在机制。方法我们已经通过在细胞和无细胞系统中建立的方案证明了载脂蛋白的微管解聚作用。研究了Apocynin对肺癌细胞A549的抑制作用。还研究了Apocynin在BALB / c小鼠模型中的抑瘤能力。将小鼠分为4组,即II组作为对照组。III-IV组小鼠也被肿瘤诱导,但是用不同剂量的阿朴西宁剂量处理,而I组小鼠保持正常。结果Apocynin对肺癌细胞而非正常肺成纤维细胞具有选择性的细胞毒性。Apocynin抑制致癌特性,包括生长,增殖(p <0.05),菌落形成(p <0.05),肺癌细胞的浸润(p <0.05)和球状形成(p <0.05)。除其他已确立的特性外,载脂蛋白还被发现是一种新颖且有效的成分,可与微管蛋白结合并解聚细胞微管网络。Apocynin介导的细胞微管解聚是触发自噬介导的凋亡细胞死亡(p <0.05)的驱动机制,继而延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。05)在肺癌细胞中。除其他已确立的特性外,载脂蛋白还被发现是一种新颖且有效的成分,可与微管蛋白结合并解聚细胞微管网络。Apocynin介导的细胞微管解聚是触发自噬介导的凋亡细胞死亡(p <0.05)的驱动机制,继而延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。05)在肺癌细胞中。除其他已确立的特性外,载脂蛋白还被发现是一种新颖且有效的成分,可与微管蛋白结合并解聚细胞微管网络。Apocynin介导的细胞微管解聚是触发自噬介导的凋亡细胞死亡(p <0.05)的驱动机制,继而延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。发现载脂蛋白是与微管蛋白结合并解聚细胞微管网络的新型有效成分。Apocynin介导的细胞微管解聚是触发自噬介导的凋亡细胞死亡(p <0.05)的驱动机制,继而延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。发现载脂蛋白是与微管蛋白结合并解聚细胞微管网络的新型有效成分。Apocynin介导的细胞微管解聚是触发自噬介导的凋亡细胞死亡(p <0.05)的驱动机制,继而延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。05)反过来又延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。05)反过来又延缓了肺癌的发展。此外,载脂蛋白还显示出抑制小鼠肺肿瘤发生的杀肿瘤特性。结论与载脂蛋白的靶向微管蛋白-微管平衡可能是巨灾细胞分解代谢过程减轻肺癌的关键调节器。因此,载脂蛋白可能是肺癌的潜在治疗剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号