当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Venom Peptides with Dual Modulatory Activity on the Voltage-Gated Sodium Channel NaV1.1 Provide Novel Leads for Development of Antiepileptic Drugs
ACS Pharmacology & Translational Science Pub Date : 2019-12-18 , DOI: 10.1021/acsptsci.9b00079 Chun Yuen Chow 1 , Yanni K.-Y. Chin 1 , Andrew A. Walker 1 , Shaodong Guo 1 , Linda V. Blomster 1 , Micaiah J. Ward 2 , Volker Herzig 1 , Darin R. Rokyta 2 , Glenn F. King 1
ACS Pharmacology & Translational Science Pub Date : 2019-12-18 , DOI: 10.1021/acsptsci.9b00079 Chun Yuen Chow 1 , Yanni K.-Y. Chin 1 , Andrew A. Walker 1 , Shaodong Guo 1 , Linda V. Blomster 1 , Micaiah J. Ward 2 , Volker Herzig 1 , Darin R. Rokyta 2 , Glenn F. King 1
Affiliation
|
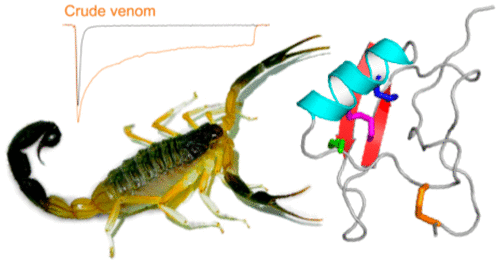
Voltage-gated sodium (NaV) channels play a fundamental role in normal neurological function, especially via the initiation and propagation of action potentials. The NaV1.1 subtype is found in inhibitory interneurons of the brain and it is essential for maintaining a balance between excitation and inhibition in neuronal networks. Heterozygous loss-of-function mutations of SCN1A, the gene encoding NaV1.1, underlie Dravet syndrome (DS), a severe pediatric epilepsy. We recently demonstrated that selective inhibition of NaV1.1 inactivation prevents seizures and premature death in a mouse model of DS. Thus, selective modulators of NaV1.1 might be useful therapeutics for treatment of DS as they target the underlying molecular deficit. Numerous scorpion-venom peptides have been shown to modulate the activity of NaV channels, but little is known about their activity at NaV1.1. Here we report the isolation, sequence, three-dimensional structure, recombinant production, and functional characterization of two peptidic modulators of NaV1.1 from venom of the buthid scorpion Hottentotta jayakari. These peptides, Hj1a and Hj2a, are potent agonists of NaV1.1 (EC50 of 17 and 32 nM, respectively), and they present dual α/β activity by modifying both the activation and inactivation properties of the channel. NMR studies of rHj1a indicate that it adopts a cystine-stabilized αβ fold similar to known scorpion toxins. Although Hj1a and Hj2a have only limited selectivity for NaV1.1, their unusual dual mode of action provides an alternative approach to the development of selective NaV1.1 modulators for the treatment of DS.
中文翻译:

在电压门控钠通道Na V 1.1上具有双重调节活性的毒肽为抗癫痫药物的开发提供了新的线索。
电压门控钠(Na V)通道在正常的神经功能中起着基本作用,尤其是通过动作电位的启动和传播。Na V 1.1亚型存在于大脑的抑制性神经元中,对于维持神经元网络的兴奋与抑制之间的平衡至关重要。编码Na V 1.1的基因SCN1A的杂合功能丧失突变是严重的小儿癫痫症Dravet综合征(DS)的基础。我们最近证明,选择性抑制Na V 1.1失活可预防DS小鼠模型中的癫痫发作和过早死亡。因此,Na V的选择性调节剂1.1可能是治疗DS的有用疗法,因为它们靶向潜在的分子缺陷。已经显示出许多蝎毒肽可调节Na V通道的活性,但对其在Na V 1.1上的活性知之甚少。在这里我们报告了从蝎蝎Hottentotta jayakari的毒液中Na V 1.1的两个肽调节剂的分离,序列,三维结构,重组生产和功能表征。这些肽Hj1a和Hj2a是Na V 1.1(EC 50分别为17 nM和32 nM),并且它们通过修改通道的激活和失活特性呈现双重α/β活性。rHj1a的NMR研究表明,它采用类似于已知蝎毒素的胱氨酸稳定化的αβ折叠。尽管Hj1a和Hj2a对Na V 1.1的选择性只有有限的限制,但它们不寻常的双重作用方式为开发用于治疗DS的选择性Na V 1.1调节剂提供了另一种方法。
更新日期:2019-12-19
中文翻译:

在电压门控钠通道Na V 1.1上具有双重调节活性的毒肽为抗癫痫药物的开发提供了新的线索。
电压门控钠(Na V)通道在正常的神经功能中起着基本作用,尤其是通过动作电位的启动和传播。Na V 1.1亚型存在于大脑的抑制性神经元中,对于维持神经元网络的兴奋与抑制之间的平衡至关重要。编码Na V 1.1的基因SCN1A的杂合功能丧失突变是严重的小儿癫痫症Dravet综合征(DS)的基础。我们最近证明,选择性抑制Na V 1.1失活可预防DS小鼠模型中的癫痫发作和过早死亡。因此,Na V的选择性调节剂1.1可能是治疗DS的有用疗法,因为它们靶向潜在的分子缺陷。已经显示出许多蝎毒肽可调节Na V通道的活性,但对其在Na V 1.1上的活性知之甚少。在这里我们报告了从蝎蝎Hottentotta jayakari的毒液中Na V 1.1的两个肽调节剂的分离,序列,三维结构,重组生产和功能表征。这些肽Hj1a和Hj2a是Na V 1.1(EC 50分别为17 nM和32 nM),并且它们通过修改通道的激活和失活特性呈现双重α/β活性。rHj1a的NMR研究表明,它采用类似于已知蝎毒素的胱氨酸稳定化的αβ折叠。尽管Hj1a和Hj2a对Na V 1.1的选择性只有有限的限制,但它们不寻常的双重作用方式为开发用于治疗DS的选择性Na V 1.1调节剂提供了另一种方法。

























 京公网安备 11010802027423号
京公网安备 11010802027423号