当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elucidating the Origin of the Electrochemical Capacity in a Proton-Based Battery HxIrO4 via Advanced Electrogravimetry.
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2019-12-18 , DOI: 10.1021/acsami.9b19349 Pierre Lemaire 1, 2, 3 , Ozlem Sel 4 , Daniel Alves Dalla Corte 1, 3 , Antonella Iadecola 3 , Hubert Perrot 4 , Jean-Marie Tarascon 1, 3
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2019-12-18 , DOI: 10.1021/acsami.9b19349 Pierre Lemaire 1, 2, 3 , Ozlem Sel 4 , Daniel Alves Dalla Corte 1, 3 , Antonella Iadecola 3 , Hubert Perrot 4 , Jean-Marie Tarascon 1, 3
Affiliation
|
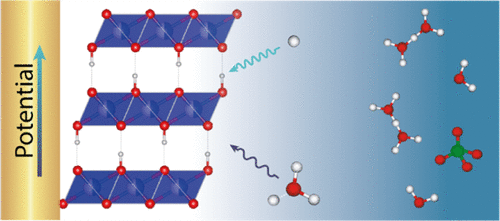
Recently, because of sustainability issues dictated by societal demands, more importance has been given to aqueous systems and especially to proton-based batteries. However, the mechanisms behind the processes leading to energy storage in such systems are still not elucidated. Under this scope, our study is structured on the selection of a model electrode material, the protonic phase HxIrO4, and the scrutiny of the interfacial processes through suitable analytical tools. Herein, we employed operando electrochemical quartz crystal microbalance (EQCM) combined with electrochemical impedance spectroscopy (EIS) to provide new insights into the mechanism intervening at the electrode-electrolyte interface. First, we demonstrated that not only the surface or near surface but the whole particle participates in the cationic redox process. Second, we proved that the contribution of the proton on the overall potential window together with the incorporation of water at low potentials solely. This is explained by the fact that water molecules permit a further insertion of protons in the material by shielding the proton charge but at the expense of the proton kinetic properties. These findings shed a new light on the importance of water molecules in the ion-insertion mechanisms taking place at the electrode-electrolyte interface of aqueous proton-based batteries. Overall, the present results further highlight the richness of the EQCM-based methods for the battery field in offering mechanistic insights that are crucial for the understanding of interfaces and charge storage in insertion compounds.
中文翻译:

通过高级电重分析阐明基于质子的电池HxIrO4中电化学容量的起源。
近来,由于社会需求所决定的可持续性问题,水系统尤其是基于质子的电池已变得更加重要。然而,仍未阐明导致此类系统中的能量存储的过程背后的机制。在此范围内,我们的研究基于模型电极材料的选择,质子相HxIrO4的选择以及通过合适的分析工具对界面过程的检查。在这里,我们采用了操作电化学石英晶体微天平(EQCM)与电化学阻抗谱(EIS)相结合,以提供对介入电极-电解质界面的机理的新见解。首先,我们证明了不仅表面或近表面,而且整个颗粒都参与了阳离子氧化还原过程。第二,我们证明了质子对整体电势窗口的贡献以及仅在低电势下掺入水。这可以通过以下事实来解释:水分子通过屏蔽质子电荷而允许质子在材料中进一步插入,但以质子动力学特性为代价。这些发现为水分子在水质子基电池的电极-电解质界面发生的离子插入机制中的重要性提供了新的思路。总体而言,本结果进一步突出了基于EQCM的电池领域方法的丰富性,提供了对理解界面和插入化合物中的电荷存储至关重要的机械见解。
更新日期:2020-01-17
中文翻译:

通过高级电重分析阐明基于质子的电池HxIrO4中电化学容量的起源。
近来,由于社会需求所决定的可持续性问题,水系统尤其是基于质子的电池已变得更加重要。然而,仍未阐明导致此类系统中的能量存储的过程背后的机制。在此范围内,我们的研究基于模型电极材料的选择,质子相HxIrO4的选择以及通过合适的分析工具对界面过程的检查。在这里,我们采用了操作电化学石英晶体微天平(EQCM)与电化学阻抗谱(EIS)相结合,以提供对介入电极-电解质界面的机理的新见解。首先,我们证明了不仅表面或近表面,而且整个颗粒都参与了阳离子氧化还原过程。第二,我们证明了质子对整体电势窗口的贡献以及仅在低电势下掺入水。这可以通过以下事实来解释:水分子通过屏蔽质子电荷而允许质子在材料中进一步插入,但以质子动力学特性为代价。这些发现为水分子在水质子基电池的电极-电解质界面发生的离子插入机制中的重要性提供了新的思路。总体而言,本结果进一步突出了基于EQCM的电池领域方法的丰富性,提供了对理解界面和插入化合物中的电荷存储至关重要的机械见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号