当前位置:
X-MOL 学术
›
Chem. Eng. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetics of Triphase Extractive Oxidative Desulfurization of Benzothiophene with Molecular Oxygen Catalyzed by HPA‐5
Chemical Engineering & Technology ( IF 2.1 ) Pub Date : 2020-02-05 , DOI: 10.1002/ceat.201900448 Johannes Claußnitzer 1 , Benjamin Bertleff 2 , Wolfgang Korth 1 , Jakob Albert 2 , Peter Wasserscheid 2 , Andreas Jess 1
Chemical Engineering & Technology ( IF 2.1 ) Pub Date : 2020-02-05 , DOI: 10.1002/ceat.201900448 Johannes Claußnitzer 1 , Benjamin Bertleff 2 , Wolfgang Korth 1 , Jakob Albert 2 , Peter Wasserscheid 2 , Andreas Jess 1
Affiliation
|
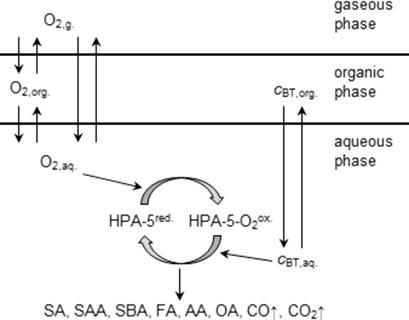
The triphasic aerobic extractive desulfurization of benzothiophene (BT) using an aqueous H8[PV5Mo7O40] solution as catalyst and O2 as oxidant was investigated. A time‐resolved analysis of all reaction products in the gas, organic and aqueous phase, is given. The organic sulfur in BT is mainly converted to sulfuric acid. Mass transport limitations can be excluded. The reaction orders are 1 with regard to BT, and 0.5 both for HPA‐5 and O2. Calculated data derived from this mechanism with a power law kinetic approach show good agreement to the experimental data for conversions below 60 %. At higher BT conversions, significant deviations are found, suggesting that acidic products formed in the BT oxidation affect the catalyst and therefore the initial kinetics of the BT oxidation.
中文翻译:

HPA-5催化分子氧对苯并噻吩进行三相萃取氧化脱硫的动力学
以H 8 [PV 5 Mo 7 O 40 ]水溶液为催化剂,O 2为氧化剂,对苯并噻吩(BT)进行了三相好氧萃取脱硫研究。给出了气相,有机相和水相中所有反应产物的时间分辨分析。BT中的有机硫主要转化为硫酸。可以排除大众运输限制。BT的反应顺序为1,HPA-5和O 2的反应顺序均为0.5。通过幂律动力学方法从该机理得出的计算数据与低于60%的转化率的实验数据显示出很好的一致性。在较高的BT转化率下,发现存在明显的偏差,表明BT氧化中形成的酸性产物会影响催化剂,从而影响BT氧化的初始动力学。
更新日期:2020-02-05
中文翻译:

HPA-5催化分子氧对苯并噻吩进行三相萃取氧化脱硫的动力学
以H 8 [PV 5 Mo 7 O 40 ]水溶液为催化剂,O 2为氧化剂,对苯并噻吩(BT)进行了三相好氧萃取脱硫研究。给出了气相,有机相和水相中所有反应产物的时间分辨分析。BT中的有机硫主要转化为硫酸。可以排除大众运输限制。BT的反应顺序为1,HPA-5和O 2的反应顺序均为0.5。通过幂律动力学方法从该机理得出的计算数据与低于60%的转化率的实验数据显示出很好的一致性。在较高的BT转化率下,发现存在明显的偏差,表明BT氧化中形成的酸性产物会影响催化剂,从而影响BT氧化的初始动力学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号