当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Imidazolidine-2,4,5- and pirimidine-2,4,6-triones - New primary pharmacophore for soluble epoxide hydrolase inhibitors with enhanced water solubility.
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2019-12-18 , DOI: 10.1016/j.bmcl.2019.126908 Vladimir Burmistrov 1 , Christophe Morisseau 2 , Vladimir D'yachenko 3 , Dmitry Karlov 4 , Gennady M Butov 3 , Bruce D Hammock 2
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2019-12-18 , DOI: 10.1016/j.bmcl.2019.126908 Vladimir Burmistrov 1 , Christophe Morisseau 2 , Vladimir D'yachenko 3 , Dmitry Karlov 4 , Gennady M Butov 3 , Bruce D Hammock 2
Affiliation
|
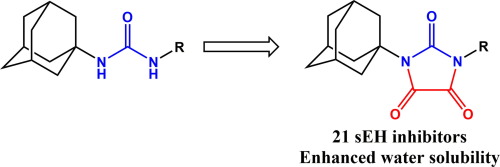
A series of inhibitors of the soluble epoxide hydrolase (sEH) containing imidazolidine-2,4,5-trione or pirimidine-2,4,6-trione has been synthesized. Inhibition potency of the described compounds ranges from 8.4 μM to 0.4 nM. The tested compounds possess higher water solubility than their preceding ureas. Molecular docking indicates new bond between the triones and the active site of sEH that in part explain the observed potency of the new pharmacophores. While less potent than the corresponding ureas, the modifications of urea group reported herein yield compounds with higher water solubility, thus permitting easier formulation.
中文翻译:

咪唑烷2,4,5-和嘧啶2,4,6-三酮-水溶性增强的可溶性环氧化物水解酶抑制剂的新主要药效基团。
合成了一系列含有咪唑烷-2,4,5-三酮或嘧啶-2,4,6-三酮的可溶性环氧化物水解酶(sEH)抑制剂。所述化合物的抑制能力为8.4μM至0.4nM。被测化合物比其先前的脲具有更高的水溶性。分子对接表明三酮与sEH的活性位点之间存在新的键,部分解释了所观察到的新药效基团的效力。尽管效力不如相应的脲,但本文报道的脲基团的修饰产生具有较高水溶性的化合物,因此使配制更容易。
更新日期:2019-12-19
中文翻译:

咪唑烷2,4,5-和嘧啶2,4,6-三酮-水溶性增强的可溶性环氧化物水解酶抑制剂的新主要药效基团。
合成了一系列含有咪唑烷-2,4,5-三酮或嘧啶-2,4,6-三酮的可溶性环氧化物水解酶(sEH)抑制剂。所述化合物的抑制能力为8.4μM至0.4nM。被测化合物比其先前的脲具有更高的水溶性。分子对接表明三酮与sEH的活性位点之间存在新的键,部分解释了所观察到的新药效基团的效力。尽管效力不如相应的脲,但本文报道的脲基团的修饰产生具有较高水溶性的化合物,因此使配制更容易。



























 京公网安备 11010802027423号
京公网安备 11010802027423号