当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An insight into the corrosion of alkali aluminoborosilicate glasses in acidic environments.
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9cp06064b Nicholas Stone-Weiss 1 , Randall E Youngman 2 , Ryan Thorpe 3 , Nicholas J Smith 2 , Eric M Pierce 4 , Ashutosh Goel 1
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9cp06064b Nicholas Stone-Weiss 1 , Randall E Youngman 2 , Ryan Thorpe 3 , Nicholas J Smith 2 , Eric M Pierce 4 , Ashutosh Goel 1
Affiliation
|
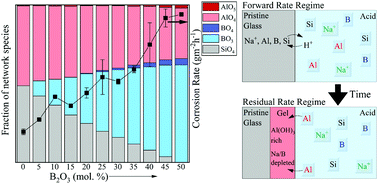
The majority of the literature on glass corrosion focuses on understanding the dissolution kinetics and mechanisms of silicate glass chemistries in the neutral-to-alkaline aqueous regime owing to its relevance in the fields of nuclear waste immobilization and biomaterials. However, understanding the corrosion of silicate-based glass chemistries over a broad composition space in the acidic pH regime is essential for glass packaging and touch screen electronic display industries. A thorough literature review on this topic reveals only a handful of studies that discuss acid corrosion of silicate glasses and their derivatives-these include only a narrow set of silicate-based glass chemistries. Although the current literature successfully explains the dissolution kinetics of glasses based upon classically understood aqueous corrosion mechanisms, more recent advancements in atomic-scale characterization techniques, have enabled a better understanding of reactions taking place directly at the pristine glass-fluid interface which has facilitated the development of a unifying model describing corrosion behavior of silicate glasses. Based on the corrosion mechanisms described and the questions raised in preceding literature, the present study focuses on understanding the corrosion mechanisms governing metaluminous (Na/Al = 1) sodium aluminoborosilicate glasses in acidic environments across a wide composition-space (ranging from SiO2-rich to B2O3-rich compositions), with particular emphasis on understanding the reactions taking place near the glass-fluid interface. Using state-of-the-art characterization techniques including nuclear magnetic resonance (NMR) spectroscopy, Rutherford backscattering, X-ray photoelectron spectroscopy (XPS) and elastic recoil detection analysis (ERDA), it has been shown that stepwise B2O3 substitutions into nepheline (NaAlSiO4) glass, although causing non-linear changes in glass structure network structural features, leads to strikingly linear increases in the forward dissolution rate at pH = 2. While the glasses undergo congruent dissolution in the forward rate regime, the residual rate regime displays evidence of preferential extraction near the glass surface (i.e., enrichment in aluminum content upon corrosion through AlO4→ Al(OH)3 evolution) implying that dissolution-re-precipitation processes may occur at the glass-fluid interface in both B2O3-rich and SiO2-rich glass compositions-albeit with vastly dissimilar reaction kinetics.
中文翻译:

深入了解碱性铝硼硅酸盐玻璃在酸性环境中的腐蚀情况。
由于其在核废料固定化和生物材料领域中的重要性,有关玻璃腐蚀的大多数文献着重于理解中性至碱性水溶液体系中硅酸盐玻璃化学物质的溶解动力学和机理。然而,对于玻璃包装和触摸屏电子显示器行业来说,了解硅酸盐基玻璃化学物质在酸性pH范围内在广泛的组成空间中的腐蚀是必不可少的。关于该主题的详尽文献评论仅显示了少数研究,讨论了硅酸盐玻璃及其衍生物的酸腐蚀-这些研究仅包括一组狭窄的基于硅酸盐的玻璃化学物质。尽管目前的文献基于经典理解的水腐蚀机理成功地解释了玻璃的溶解动力学,原子尺度表征技术的最新进展使人们能够更好地理解直接在原始玻璃-流体界面上发生的反应,这促进了描述硅酸盐玻璃腐蚀行为的统一模型的发展。基于所描述的腐蚀机理和先前文献中提出的问题,本研究着重于了解在酸性环境中跨较大组成空间(从富含SiO2的溶液)控制金属铝(Na / Al = 1)铝硼硅酸钠玻璃的腐蚀机理。 (富含B 2 O 3的组合物),特别着重于了解在玻璃流体界面附近发生的反应。使用包括核磁共振(NMR)光谱在内的最新表征技术,
更新日期:2020-01-08
中文翻译:

深入了解碱性铝硼硅酸盐玻璃在酸性环境中的腐蚀情况。
由于其在核废料固定化和生物材料领域中的重要性,有关玻璃腐蚀的大多数文献着重于理解中性至碱性水溶液体系中硅酸盐玻璃化学物质的溶解动力学和机理。然而,对于玻璃包装和触摸屏电子显示器行业来说,了解硅酸盐基玻璃化学物质在酸性pH范围内在广泛的组成空间中的腐蚀是必不可少的。关于该主题的详尽文献评论仅显示了少数研究,讨论了硅酸盐玻璃及其衍生物的酸腐蚀-这些研究仅包括一组狭窄的基于硅酸盐的玻璃化学物质。尽管目前的文献基于经典理解的水腐蚀机理成功地解释了玻璃的溶解动力学,原子尺度表征技术的最新进展使人们能够更好地理解直接在原始玻璃-流体界面上发生的反应,这促进了描述硅酸盐玻璃腐蚀行为的统一模型的发展。基于所描述的腐蚀机理和先前文献中提出的问题,本研究着重于了解在酸性环境中跨较大组成空间(从富含SiO2的溶液)控制金属铝(Na / Al = 1)铝硼硅酸钠玻璃的腐蚀机理。 (富含B 2 O 3的组合物),特别着重于了解在玻璃流体界面附近发生的反应。使用包括核磁共振(NMR)光谱在内的最新表征技术,



























 京公网安备 11010802027423号
京公网安备 11010802027423号