Journal of Catalysis ( IF 7.3 ) Pub Date : 2019-12-18 , DOI: 10.1016/j.jcat.2019.11.036 Wenqiang Yang , Rajadurai Vijay Solomon , Jianmin Lu , Osman Mamun , Jesse Q. Bond , Andreas Heyden
|
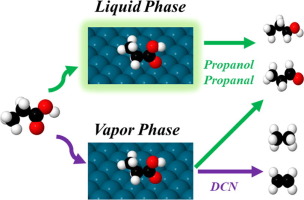
Microkinetic models based on first principles calculations have been developed for the vapor and liquid phase hydrodeoxygenation of propionic acid over a Pt (1 1 1) surface. Calculations suggest that decarboxylation does not occur at an appreciable rate. In the vapor phase, decarbonylation products, propanal and propanol are all produced at similar rates. However, in both liquid water and 1,4-dioxane, propanol and propanal are favored over decarbonylation products. While a condensed phase can shift the reaction rate and selectivity significantly, the dominant pathways towards the various products are hardly affected. Only for propanal production do we observe a shift in mechanism. At 473 K, the propionic acid conversion rate is increased by one order of magnitude in liquid 1,4-dioxane relative to the gas phase. In liquid water, the conversion rate is similar to the vapor phase since adsorbed propionic acid blocks a large fraction of the surface sites.
中文翻译:

阐明丙酸在气相和液相中在Pt(1 1 1)表面上加氢脱氧的机理
已经开发了基于第一原理计算的微动力学模型,用于在Pt(1 1 1)表面上进行丙酸的汽相和液相加氢脱氧。计算表明脱羧不会以明显的速率发生。在气相中,脱羰产物,丙醛和丙醇都以相似的速率产生。但是,在液态水和1,4-二恶烷中,丙醇和丙醛均优于脱羰基产物。虽然冷凝相可以显着改变反应速率和选择性,但几乎不影响通向各种产物的主要途径。仅对于丙烷生产,我们观察到机理发生了变化。在473 K下,液体1,4-二恶烷中的丙酸转化率相对于气相增加了一个数量级。在液态水中



























 京公网安备 11010802027423号
京公网安备 11010802027423号