Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantifying the Extent of Hydration of a Surface-Bound Peptide Using Neutron Reflectometry.
Langmuir ( IF 3.9 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.langmuir.9b02559 Whitney A Fies 1 , Jeremy T First 1 , Jason W Dugger 2 , Mathieu Doucet 3 , James F Browning 3 , Lauren J Webb 1
Langmuir ( IF 3.9 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.langmuir.9b02559 Whitney A Fies 1 , Jeremy T First 1 , Jason W Dugger 2 , Mathieu Doucet 3 , James F Browning 3 , Lauren J Webb 1
Affiliation
|
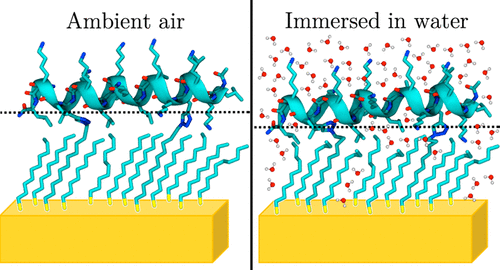
Establishing how water, or the absence of water, affects the structure, dynamics, and function of proteins in contact with inorganic surfaces is critical to developing successful protein immobilization strategies. In the present article, the quantity of water hydrating a monolayer of helical peptides covalently attached to self-assembled monolayers (SAMs) of alkyl thiols on Au was measured using neutron reflectometry (NR). The peptide sequence was composed of repeating LLKK units in which the leucines were aligned to face the SAM. When immersed in water, NR measured 2.7 ± 0.9 water molecules per thiol in the SAM layer and between 75 ± 13 and 111 ± 13 waters around each peptide. The quantity of water in the SAM was nearly twice that measured prior to peptide functionalization, suggesting that the peptide disrupted the structure of the SAM. To identify the location of water molecules around the peptide, we compared our NR data to previously published molecular dynamics simulations of the same peptide on a hydrophobic SAM in water, revealing that 49 ± 5 of 95 ± 8 total nearby water molecules were directly hydrogen-bound to the peptide. Finally, we show that immersing the peptide in water compressed its structure into the SAM surface. Together, these results demonstrate that there is sufficient water to fully hydrate a surface-bound peptide even at hydrophobic interfaces. Given the critical role that water plays in biomolecular structure and function, these results are expected to be informative for a broad array of applications involving proteins at the bio/abio interface.
中文翻译:

使用中子反射计量化表面结合肽的水合作用程度。
建立水或不存在水如何影响与无机表面接触的蛋白质的结构,动力学和功能对于开发成功的蛋白质固定策略至关重要。在本文中,使用中子反射法(NR)测量了水化与烷基硫醇的自组装单分子层(SAMs)共价连接的螺旋肽单分子层水合的水量。肽序列由重复的LLKK单位组成,其中的亮氨酸比对着SAM。浸入水中时,NR在SAM层中每个巯基上测得2.7±0.9个水分子,每个肽周围的水量在75±13到111±13之间。SAM中的水量几乎是肽功能化之前测量的水量的两倍,表明该肽破坏了SAM的结构。为了确定肽周围水分子的位置,我们将NR数据与之前发表的同一肽在水中疏水SAM上的分子动力学模拟进行了比较,发现在95±8个附近的水分子中,有49±5直接是氢-结合到肽上。最后,我们表明将肽浸入水中可将其结构压缩到SAM表面中。总之,这些结果表明即使在疏水性界面处也有足够的水使表面结合的肽完全水合。考虑到水在生物分子的结构和功能中所起的关键作用,这些结果有望为涉及生物/非生物界面蛋白质的广泛应用提供有益的信息。我们将NR数据与先前在水中的疏水性SAM上发布的同一肽的分子动力学模拟进行了比较,发现在95±8的总附近水分子中有49±5直接与氢结合。最后,我们表明将肽浸入水中可将其结构压缩到SAM表面中。总之,这些结果表明,即使在疏水界面处,也有足够的水使表面结合的肽完全水合。考虑到水在生物分子的结构和功能中所起的关键作用,这些结果有望为涉及生物/非生物界面蛋白质的广泛应用提供有益的信息。我们将NR数据与先前在水中的疏水性SAM上发布的同一肽的分子动力学模拟进行了比较,发现在95±8的总附近水分子中有49±5直接与氢结合。最后,我们表明将肽浸入水中可将其结构压缩到SAM表面中。总之,这些结果表明即使在疏水性界面处也有足够的水使表面结合的肽完全水合。考虑到水在生物分子的结构和功能中所起的关键作用,这些结果有望为涉及生物/非生物界面蛋白质的广泛应用提供有益的信息。最后,我们表明将肽浸入水中可将其结构压缩到SAM表面中。总之,这些结果表明即使在疏水性界面处也有足够的水使表面结合的肽完全水合。考虑到水在生物分子的结构和功能中所起的关键作用,这些结果有望为涉及生物/非生物界面蛋白质的广泛应用提供有益的信息。最后,我们表明将肽浸入水中可将其结构压缩到SAM表面中。总之,这些结果表明即使在疏水性界面处也有足够的水使表面结合的肽完全水合。考虑到水在生物分子的结构和功能中所起的关键作用,这些结果有望为涉及生物/非生物界面蛋白质的广泛应用提供有益的信息。
更新日期:2020-01-07
中文翻译:

使用中子反射计量化表面结合肽的水合作用程度。
建立水或不存在水如何影响与无机表面接触的蛋白质的结构,动力学和功能对于开发成功的蛋白质固定策略至关重要。在本文中,使用中子反射法(NR)测量了水化与烷基硫醇的自组装单分子层(SAMs)共价连接的螺旋肽单分子层水合的水量。肽序列由重复的LLKK单位组成,其中的亮氨酸比对着SAM。浸入水中时,NR在SAM层中每个巯基上测得2.7±0.9个水分子,每个肽周围的水量在75±13到111±13之间。SAM中的水量几乎是肽功能化之前测量的水量的两倍,表明该肽破坏了SAM的结构。为了确定肽周围水分子的位置,我们将NR数据与之前发表的同一肽在水中疏水SAM上的分子动力学模拟进行了比较,发现在95±8个附近的水分子中,有49±5直接是氢-结合到肽上。最后,我们表明将肽浸入水中可将其结构压缩到SAM表面中。总之,这些结果表明即使在疏水性界面处也有足够的水使表面结合的肽完全水合。考虑到水在生物分子的结构和功能中所起的关键作用,这些结果有望为涉及生物/非生物界面蛋白质的广泛应用提供有益的信息。我们将NR数据与先前在水中的疏水性SAM上发布的同一肽的分子动力学模拟进行了比较,发现在95±8的总附近水分子中有49±5直接与氢结合。最后,我们表明将肽浸入水中可将其结构压缩到SAM表面中。总之,这些结果表明,即使在疏水界面处,也有足够的水使表面结合的肽完全水合。考虑到水在生物分子的结构和功能中所起的关键作用,这些结果有望为涉及生物/非生物界面蛋白质的广泛应用提供有益的信息。我们将NR数据与先前在水中的疏水性SAM上发布的同一肽的分子动力学模拟进行了比较,发现在95±8的总附近水分子中有49±5直接与氢结合。最后,我们表明将肽浸入水中可将其结构压缩到SAM表面中。总之,这些结果表明即使在疏水性界面处也有足够的水使表面结合的肽完全水合。考虑到水在生物分子的结构和功能中所起的关键作用,这些结果有望为涉及生物/非生物界面蛋白质的广泛应用提供有益的信息。最后,我们表明将肽浸入水中可将其结构压缩到SAM表面中。总之,这些结果表明即使在疏水性界面处也有足够的水使表面结合的肽完全水合。考虑到水在生物分子的结构和功能中所起的关键作用,这些结果有望为涉及生物/非生物界面蛋白质的广泛应用提供有益的信息。最后,我们表明将肽浸入水中可将其结构压缩到SAM表面中。总之,这些结果表明即使在疏水性界面处也有足够的水使表面结合的肽完全水合。考虑到水在生物分子的结构和功能中所起的关键作用,这些结果有望为涉及生物/非生物界面蛋白质的广泛应用提供有益的信息。



























 京公网安备 11010802027423号
京公网安备 11010802027423号