当前位置:
X-MOL 学术
›
PLOS Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Glioblastoma cells vampirize WNT from neurons and trigger a JNK/MMP signaling loop that enhances glioblastoma progression and neurodegeneration.
PLOS Biology ( IF 9.8 ) Pub Date : 2019-12-17 , DOI: 10.1371/journal.pbio.3000545 Marta Portela 1 , Varun Venkataramani 2, 3, 4 , Natasha Fahey-Lozano 1 , Esther Seco 1 , Maria Losada-Perez 1 , Frank Winkler 2, 3 , Sergio Casas-Tintó 1
PLOS Biology ( IF 9.8 ) Pub Date : 2019-12-17 , DOI: 10.1371/journal.pbio.3000545 Marta Portela 1 , Varun Venkataramani 2, 3, 4 , Natasha Fahey-Lozano 1 , Esther Seco 1 , Maria Losada-Perez 1 , Frank Winkler 2, 3 , Sergio Casas-Tintó 1
Affiliation
|
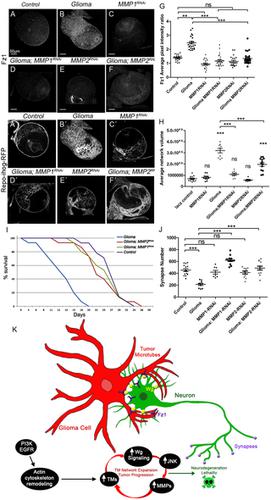
Glioblastoma (GB) is the most lethal brain tumor, and Wingless (Wg)-related integration site (WNT) pathway activation in these tumors is associated with a poor prognosis. Clinically, the disease is characterized by progressive neurological deficits. However, whether these symptoms result from direct or indirect damage to neurons is still unresolved. Using Drosophila and primary xenografts as models of human GB, we describe, here, a mechanism that leads to activation of WNT signaling (Wg in Drosophila) in tumor cells. GB cells display a network of tumor microtubes (TMs) that enwrap neurons, accumulate Wg receptor Frizzled1 (Fz1), and, thereby, deplete Wg from neurons, causing neurodegeneration. We have defined this process as "vampirization." Furthermore, GB cells establish a positive feedback loop to promote their expansion, in which the Wg pathway activates cJun N-terminal kinase (JNK) in GB cells, and, in turn, JNK signaling leads to the post-transcriptional up-regulation and accumulation of matrix metalloproteinases (MMPs), which facilitate TMs' infiltration throughout the brain, TMs' network expansion, and further Wg depletion from neurons. Consequently, GB cells proliferate because of the activation of the Wg signaling target, β-catenin, and neurons degenerate because of Wg signaling extinction. Our findings reveal a molecular mechanism for TM production, infiltration, and maintenance that can explain both neuron-dependent tumor progression and also the neural decay associated with GB.
中文翻译:

胶质母细胞瘤细胞使来自神经元的WNT吸血并触发JNK / MMP信号回路,从而增强了胶质母细胞瘤的进展和神经变性。
胶质母细胞瘤(GB)是最致命的脑肿瘤,这些肿瘤中Wingless(Wg)相关的整合位点(WNT)途径活化与不良预后相关。临床上,该疾病的特征是进行性神经功能缺损。但是,这些症状是由直接还是间接损害神经元引起的仍未解决。使用果蝇和原代异种移植作为人类GB的模型,我们在这里描述了一种机制,该机制导致肿瘤细胞中WNT信号的激活(果蝇中的Wg)激活。GB细胞显示出包裹神经元,积累Wg受体Frizzled1(Fz1)的肿瘤微管(TM)网络,从而消耗神经元中的Wg,从而导致神经退行性变。我们将这一过程定义为“吸血”。此外,GB细胞建立正反馈回路以促进其扩展,其中Wg途径激活GB细胞中的cJun N末端激酶(JNK),JNK信号转导导致转录后上调和基质金属蛋白酶(MMP)的积累,从而促进TM贯穿整个T细胞的浸润。大脑,TM的网络扩展以及神经元中Wg的进一步消耗。因此,GB细胞由于Wg信号靶的激活而增殖,β-catenin和神经元由于Wg信号的灭绝而退化。我们的发现揭示了TM产生,浸润和维持的分子机制,可以解释神经元依赖性肿瘤的进展以及与GB相关的神经衰变。JNK信号传导导致转录后上调和基质金属蛋白酶(MMP)积累,从而促进TMs在整个大脑中的浸润,TMs的网络扩展以及神经元中Wg的进一步消耗。因此,GB细胞由于Wg信号靶的激活而增殖,β-catenin和神经元由于Wg信号的灭绝而退化。我们的发现揭示了TM产生,浸润和维持的分子机制,可以解释神经元依赖性肿瘤的进展以及与GB相关的神经衰变。JNK信号传导导致转录后上调和基质金属蛋白酶(MMP)积累,从而促进TMs在整个大脑中的浸润,TMs的网络扩展以及神经元中Wg的进一步消耗。因此,GB细胞由于Wg信号靶的激活而增殖,β-catenin和神经元由于Wg信号的灭绝而退化。我们的发现揭示了TM产生,浸润和维持的分子机制,可以解释神经元依赖性肿瘤的进展以及与GB相关的神经衰变。Wg信号的灭绝导致神经元退化。我们的发现揭示了TM产生,浸润和维持的分子机制,可以解释神经元依赖性肿瘤的进展以及与GB相关的神经衰变。Wg信号的灭绝导致神经元退化。我们的发现揭示了TM产生,浸润和维持的分子机制,可以解释神经元依赖性肿瘤的进展以及与GB相关的神经衰变。
更新日期:2019-12-18
中文翻译:

胶质母细胞瘤细胞使来自神经元的WNT吸血并触发JNK / MMP信号回路,从而增强了胶质母细胞瘤的进展和神经变性。
胶质母细胞瘤(GB)是最致命的脑肿瘤,这些肿瘤中Wingless(Wg)相关的整合位点(WNT)途径活化与不良预后相关。临床上,该疾病的特征是进行性神经功能缺损。但是,这些症状是由直接还是间接损害神经元引起的仍未解决。使用果蝇和原代异种移植作为人类GB的模型,我们在这里描述了一种机制,该机制导致肿瘤细胞中WNT信号的激活(果蝇中的Wg)激活。GB细胞显示出包裹神经元,积累Wg受体Frizzled1(Fz1)的肿瘤微管(TM)网络,从而消耗神经元中的Wg,从而导致神经退行性变。我们将这一过程定义为“吸血”。此外,GB细胞建立正反馈回路以促进其扩展,其中Wg途径激活GB细胞中的cJun N末端激酶(JNK),JNK信号转导导致转录后上调和基质金属蛋白酶(MMP)的积累,从而促进TM贯穿整个T细胞的浸润。大脑,TM的网络扩展以及神经元中Wg的进一步消耗。因此,GB细胞由于Wg信号靶的激活而增殖,β-catenin和神经元由于Wg信号的灭绝而退化。我们的发现揭示了TM产生,浸润和维持的分子机制,可以解释神经元依赖性肿瘤的进展以及与GB相关的神经衰变。JNK信号传导导致转录后上调和基质金属蛋白酶(MMP)积累,从而促进TMs在整个大脑中的浸润,TMs的网络扩展以及神经元中Wg的进一步消耗。因此,GB细胞由于Wg信号靶的激活而增殖,β-catenin和神经元由于Wg信号的灭绝而退化。我们的发现揭示了TM产生,浸润和维持的分子机制,可以解释神经元依赖性肿瘤的进展以及与GB相关的神经衰变。JNK信号传导导致转录后上调和基质金属蛋白酶(MMP)积累,从而促进TMs在整个大脑中的浸润,TMs的网络扩展以及神经元中Wg的进一步消耗。因此,GB细胞由于Wg信号靶的激活而增殖,β-catenin和神经元由于Wg信号的灭绝而退化。我们的发现揭示了TM产生,浸润和维持的分子机制,可以解释神经元依赖性肿瘤的进展以及与GB相关的神经衰变。Wg信号的灭绝导致神经元退化。我们的发现揭示了TM产生,浸润和维持的分子机制,可以解释神经元依赖性肿瘤的进展以及与GB相关的神经衰变。Wg信号的灭绝导致神经元退化。我们的发现揭示了TM产生,浸润和维持的分子机制,可以解释神经元依赖性肿瘤的进展以及与GB相关的神经衰变。


























 京公网安备 11010802027423号
京公网安备 11010802027423号