Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Molecular Architecture of the Human γ-Tubulin Ring Complex.
Cell ( IF 64.5 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.cell.2019.12.007 Michal Wieczorek 1 , Linas Urnavicius 2 , Shih-Chieh Ti 1 , Kelly R Molloy 3 , Brian T Chait 3 , Tarun M Kapoor 1
Cell ( IF 64.5 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.cell.2019.12.007 Michal Wieczorek 1 , Linas Urnavicius 2 , Shih-Chieh Ti 1 , Kelly R Molloy 3 , Brian T Chait 3 , Tarun M Kapoor 1
Affiliation
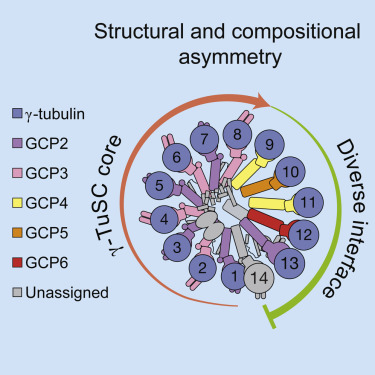
|
The γ-tubulin ring complex (γ-TuRC) is an essential regulator of centrosomal and acentrosomal microtubule formation, yet its structure is not known. Here, we present a cryo-EM reconstruction of the native human γ-TuRC at ∼3.8 Å resolution, revealing an asymmetric, cone-shaped structure. Pseudo-atomic models indicate that GCP4, GCP5, and GCP6 form distinct Y-shaped assemblies that structurally mimic GCP2/GCP3 subcomplexes distal to the γ-TuRC "seam." We also identify an unanticipated structural bridge that includes an actin-like protein and spans the γ-TuRC lumen. Despite its asymmetric architecture, the γ-TuRC arranges γ-tubulins into a helical geometry poised to nucleate microtubules. Diversity in the γ-TuRC subunits introduces large (>100,000 Å2) surfaces in the complex that allow for interactions with different regulatory factors. The observed compositional complexity of the γ-TuRC could self-regulate its assembly into a cone-shaped structure to control microtubule formation across diverse contexts, e.g., within biological condensates or alongside existing filaments.
中文翻译:

人γ-管蛋白环复合物的不对称分子结构。
γ-微管蛋白环复合物(γ-TuRC)是中心体和中心体微管形成的重要调节剂,但其结构尚不清楚。在这里,我们提出了以约3.8Å分辨率对天然人γ-TuRC进行冷冻-EM重建的方法,揭示了一种不对称的锥形结构。伪原子模型表明,GCP4,GCP5和GCP6形成了不同的Y形组件,这些组件在结构上模仿了GCP2 / GCP3在γ-TuRC“接缝”远端的子复合体。我们还确定了一个意想不到的结构桥,其中包括肌动蛋白样蛋白,跨过γ-TuRC内腔。尽管具有非对称结构,但γ-TuRC仍将γ-微管蛋白排列成螺旋形,从而使微管成核。γ-TuRC亚基的多样性会在复合物中引入较大的表面(> 100,000Å2),从而可以与不同的调节因子相互作用。
更新日期:2019-12-18
中文翻译:

人γ-管蛋白环复合物的不对称分子结构。
γ-微管蛋白环复合物(γ-TuRC)是中心体和中心体微管形成的重要调节剂,但其结构尚不清楚。在这里,我们提出了以约3.8Å分辨率对天然人γ-TuRC进行冷冻-EM重建的方法,揭示了一种不对称的锥形结构。伪原子模型表明,GCP4,GCP5和GCP6形成了不同的Y形组件,这些组件在结构上模仿了GCP2 / GCP3在γ-TuRC“接缝”远端的子复合体。我们还确定了一个意想不到的结构桥,其中包括肌动蛋白样蛋白,跨过γ-TuRC内腔。尽管具有非对称结构,但γ-TuRC仍将γ-微管蛋白排列成螺旋形,从而使微管成核。γ-TuRC亚基的多样性会在复合物中引入较大的表面(> 100,000Å2),从而可以与不同的调节因子相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号