当前位置:
X-MOL 学术
›
Lancet Diabetes Endocrinol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study.
The Lancet Diabetes & Endocrinology ( IF 44.5 ) Pub Date : 2020-01-01 , DOI: 10.1016/s2213-8587(19)30384-5 Hiddo J L Heerspink , Avraham Karasik , Marcus Thuresson , Cheli Melzer-Cohen , Gabriel Chodick , Kamlesh Khunti , John P H Wilding , Luis Alberto Garcia Rodriguez , Lucia Cea-Soriano , Shun Kohsaka , Antonio Nicolucci , Giuseppe Lucisano , Fang-Ju Lin , Chih-Yuan Wang , Eric Wittbrodt , Peter Fenici , Mikhail Kosiborod
The Lancet Diabetes & Endocrinology ( IF 44.5 ) Pub Date : 2020-01-01 , DOI: 10.1016/s2213-8587(19)30384-5 Hiddo J L Heerspink , Avraham Karasik , Marcus Thuresson , Cheli Melzer-Cohen , Gabriel Chodick , Kamlesh Khunti , John P H Wilding , Luis Alberto Garcia Rodriguez , Lucia Cea-Soriano , Shun Kohsaka , Antonio Nicolucci , Giuseppe Lucisano , Fang-Ju Lin , Chih-Yuan Wang , Eric Wittbrodt , Peter Fenici , Mikhail Kosiborod
|
BACKGROUND
Cardiovascular and kidney outcome trials have shown that sodium-glucose co-transporter-2 (SGLT2) inhibitors slow progression of chronic kidney disease in patients with type 2 diabetes with or without chronic kidney disease. The aim of this study was to assess whether these benefits extend to patients with type 2 diabetes treated in routine clinical practice.
METHODS
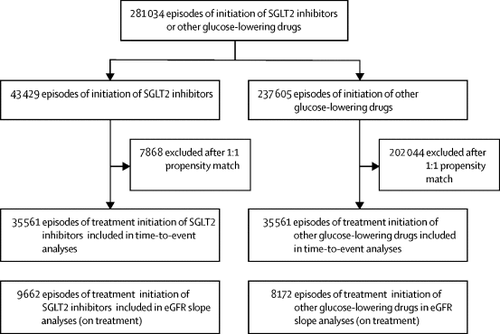
CVD-REAL 3 was a multinational observational cohort study in which new users of SGLT2 inhibitors and other glucose-lowering drugs with measurements of estimated glomerular filtration rate (eGFR) before and after (within 180 days) initiation were identified via claims, medical records, and national registries in Israel, Italy, Japan, Taiwan, and the UK. Propensity scores for SGLT2 inhibitor initiation were developed in each country, with 1:1 matching with initiators of other glucose-lowering drugs. Propensity score included (in addition to other clinical and demographic variables) baseline eGFR and eGFR slope before SGLT2 inhibitor or other glucose-lowering drug initiation. The main outcome measure was rate of eGFR decline (slope) calculated with a linear mixed regression model. Differences in eGFR slope between SGLT2 inhibitors and other glucose-lowering drugs were calculated and pooled. We also assessed a composite outcome of 50% eGFR decline or end-stage kidney disease.
FINDINGS
After propensity matching, there were 35 561 episodes of treatment initiation in each group, from 65 231 individual patients. Dapagliflozin, empagliflozin, canagliflozin, ipragliflozin, tofogliflozin, and luseogliflozin accounted for 57·9%, 34·1%, 5·7%, 1·4%, 0·5%, and 0·4% of SGLT2 inhibitor initiation episodes, respectively. At baseline, 29 363 (41·3%) of 71 122 initiations were in women, mean age was 61·3 years, mean HbA1c was 72 mmol/mol (8·71%), and mean eGFR was 90·7 mL/min per 1·73 m2. During follow-up, SGLT2 inhibitor initiation was associated with reduced eGFR decline (difference in slope for SGLT2 inhibitors vs other glucose-lowering drugs 1·53 mL/min per 1·73 m2 per year, 95% CI 1·34-1·72, p<0·0001). During a mean follow-up of 14·9 months, 351 composite kidney outcomes occurred: 114 (3·0 events per 10 000 patient-years) among initiators of SGLT2 inhibitors and 237 (6·3 events per 10 000 patient-years) among initiators of other glucose-lowering drugs (hazard ratio 0·49, 95% CI 0·35-0·67; p<0·0001). These findings were consistent across countries (pheterogeneity 0·10) and prespecified subgroups.
INTERPRETATION
In this large, international, real-world study of patients with type 2 diabetes, initiation of SGLT2 inhibitor therapy was associated with a slower rate of kidney function decline and lower risk of major kidney events compared with initiation of other glucose-lowering drugs. These data suggest that the benefits of SGLT2 inhibitors on kidney function identified in clinical trials seem to be largely generalisable to clinical practice.
FUNDING
AstraZeneca.
更新日期:2019-12-18


























 京公网安备 11010802027423号
京公网安备 11010802027423号