当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Helically Chiral Aromatics: The Synthesis of Helicenes by [2 + 2 + 2] Cycloisomerization of π-Electron Systems.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2019-12-13 , DOI: 10.1021/acs.accounts.9b00364 Irena G Stará 1 , Ivo Starý 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2019-12-13 , DOI: 10.1021/acs.accounts.9b00364 Irena G Stará 1 , Ivo Starý 1
Affiliation
|
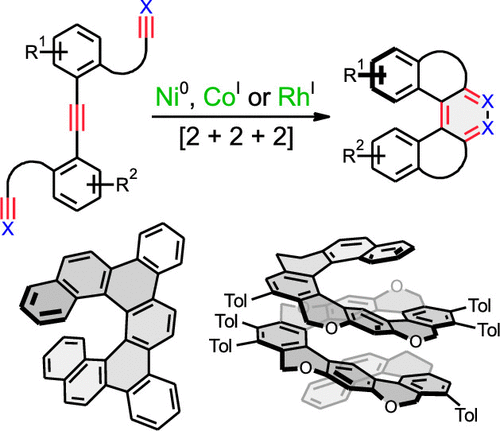
Advanced molecular nanocarbons are now in the spotlight reflecting the basic discoveries of fullerenes, carbon nanotubes, and graphene. This research area includes also the chemistry, physics, and nanoscience of nonplanar polycyclic hydrocarbons, many of which exhibit helical chirality, such as iconic helicenes and their congeners. The combination of unique π-electron systems with the chirality phenomenon makes them highly attractive in various fields of science. Helicenes are polyaromatic compounds that are composed of all-angularly annulated benzene units, but other (hetero)cycles can also be embedded into their backbone. Even though they do not contain any stereogenic center, they are inherently chiral owing to the helical shape they adopt. Hexahelicene and higher homologues are conformationally stable within a reasonable range of temperatures and, therefore, can be obtained in an enantiopure form through a racemate resolution or asymmetric synthesis. An amazing array of synthetic methods for their preparation has been developed, but only a few of them have passed the tough scrutiny to be general, robust and practical methods such as traditional photocyclodehydrogenation of diaryl olefins and recently developed transition-metal-catalyzed [2 + 2 + 2] cycloisomerization of π-electron systems, which is discussed in this Account. Alkyne [2 + 2 + 2] cycloisomerization is a highly exergonic process and is therefore suitable for forming the strained helicene backbone, three (or more) cycles of which are closed in a single operation. The typical starting materials are aromatic triynes (optionally cyanodiynes or ynedinitriles) or tetraynes with diynes that undergo intramolecular or intermolecular cyclization, respectively, catalyzed by various complexes mainly of Ni0, CoI, or RhI. Utilizing this synthetic methodology, various [5]-, [6]-, [7]-, [9]-, [11]-, [13]-, [16]-, [17]-, and [19]helicenes or their congeners, including functionalized derivatives, can be effectively prepared. Moreover, asymmetric synthesis (both catalytic and stoichiometric) of nonracemic helicenes has already been demonstrated. It relies on [2 + 2 + 2] cycloisomerization of centrally chiral triynes followed by an asymmetric transformation of the first order (controlled by the 1,3-allylic-type strain) or on enantioselective [2 + 2 + 2] cycloisomerization of alkynes catalyzed by chiral complexes mainly of Ni0 or RhI. Intriguingly, advanced helical architectures were formed such as the longest helicenes (up to oxa[19]helicene by closing 12 rings in a single synthetic operation) or laterally extended helicenes (e.g., pyreno[7]helicenes). Utilizing the aforementioned synthetic methodology, the tailor-made helical molecular nanocarbons are now better accessible to be applied in enantioselective catalysis, chirality sensing, spintronics (based on chirality induced spin selectivity), chiroptics (to produce circularly polarized light emission), organic/molecular electronics, or chiral single molecule devices.
中文翻译:

螺旋手性芳烃:通过[2 + 2 + 2]π电子体系的环异构化反应合成螺旋。
如今,先进的分子纳米碳已经成为人们关注的焦点,反映了富勒烯,碳纳米管和石墨烯的基本发现。该研究领域还包括非平面多环烃的化学,物理和纳米科学,其中许多具有螺旋手性,例如标志性螺旋烯及其同类物。独特的π电子系统与手性现象的结合,使它们在科学的各个领域都具有很高的吸引力。螺旋烯是由全角环苯单元组成的多芳族化合物,但是其他(杂)环也可以嵌入其骨架中。即使它们不包含任何立体异构中心,由于其采用的螺旋形状,它们固有地是手性的。六方烯和更高的同系物在合理的温度范围内是构象稳定的,因此可以通过外消旋体拆分或不对称合成以对映体纯形式获得。已经开发了一系列惊人的合成方法,但其中只有少数经过严格的审查,成为常规,稳健和实用的方法,例如传统的二芳基烯烃的光环脱氢和最近开发的过渡金属催化的[2 + 2 + 2]π电子系统的环异构化,将在本报告中进行讨论。炔烃[2 + 2 + 2]环异构化是一种高能过程,因此适合于形成应变的螺旋烯骨架,在一次操作中关闭三个(或更多个)循环。典型的起始原料是芳香族三炔(可选的氰基二炔或炔腈)或四炔与二炔,它们分别通过主要由Ni0,CoI或RhI形成的各种配合物进行分子内或分子间环化。利用这种综合方法,可以使用各种[5]-,[6]-,[7]-,[9]-,[11]-,[13]-,[16]-,[17]-和[19]可以有效地制备螺旋烯或其同类物,包括功能化衍生物。而且,已经证明了非外消旋螺旋烯的不对称合成(催化的和化学计量的)。它依赖于中央手性三炔的[2 + 2 + 2]环异构化,然后是一阶的不对称转化(由1控制,3-烯丙基型菌株)或主要由Ni0或RhI的手性络合物催化的炔烃的对映选择性[2 + 2 + 2]环异构化。有趣的是,形成了高级的螺旋结构,例如最长的螺旋(通过在一次合成操作中关闭12个环,最多达到oxa [19]螺旋)或横向延伸的螺旋(例如,pyreno [7]螺旋)。利用上述合成方法,可以更好地利用量身定制的螺旋分子纳米碳,用于对映选择性催化,手性传感,自旋电子学(基于手性引起的自旋选择性),手性学(产生圆偏振光发射),有机/分子电子或手性单分子设备。形成了先进的螺旋结构,例如最长的螺旋烯(通过在一次合成操作中关闭12个环,最多达到oxa [19]螺旋烯)或横向延伸的螺旋烯(例如,pyreno [7]螺旋烯)。利用上述合成方法,可以更好地利用量身定制的螺旋分子纳米碳,用于对映选择性催化,手性传感,自旋电子学(基于手性引起的自旋选择性),手性学(产生圆偏振光发射),有机/分子电子或手性单分子设备。形成了先进的螺旋结构,例如最长的螺旋烯(通过在一次合成操作中关闭12个环,最多达到oxa [19]螺旋烯)或横向延伸的螺旋烯(例如,pyreno [7]螺旋烯)。利用上述合成方法,可以更好地利用量身定制的螺旋分子纳米碳,用于对映选择性催化,手性传感,自旋电子学(基于手性引起的自旋选择性),手性学(产生圆偏振光发射),有机/分子电子或手性单分子设备。
更新日期:2019-12-17
中文翻译:

螺旋手性芳烃:通过[2 + 2 + 2]π电子体系的环异构化反应合成螺旋。
如今,先进的分子纳米碳已经成为人们关注的焦点,反映了富勒烯,碳纳米管和石墨烯的基本发现。该研究领域还包括非平面多环烃的化学,物理和纳米科学,其中许多具有螺旋手性,例如标志性螺旋烯及其同类物。独特的π电子系统与手性现象的结合,使它们在科学的各个领域都具有很高的吸引力。螺旋烯是由全角环苯单元组成的多芳族化合物,但是其他(杂)环也可以嵌入其骨架中。即使它们不包含任何立体异构中心,由于其采用的螺旋形状,它们固有地是手性的。六方烯和更高的同系物在合理的温度范围内是构象稳定的,因此可以通过外消旋体拆分或不对称合成以对映体纯形式获得。已经开发了一系列惊人的合成方法,但其中只有少数经过严格的审查,成为常规,稳健和实用的方法,例如传统的二芳基烯烃的光环脱氢和最近开发的过渡金属催化的[2 + 2 + 2]π电子系统的环异构化,将在本报告中进行讨论。炔烃[2 + 2 + 2]环异构化是一种高能过程,因此适合于形成应变的螺旋烯骨架,在一次操作中关闭三个(或更多个)循环。典型的起始原料是芳香族三炔(可选的氰基二炔或炔腈)或四炔与二炔,它们分别通过主要由Ni0,CoI或RhI形成的各种配合物进行分子内或分子间环化。利用这种综合方法,可以使用各种[5]-,[6]-,[7]-,[9]-,[11]-,[13]-,[16]-,[17]-和[19]可以有效地制备螺旋烯或其同类物,包括功能化衍生物。而且,已经证明了非外消旋螺旋烯的不对称合成(催化的和化学计量的)。它依赖于中央手性三炔的[2 + 2 + 2]环异构化,然后是一阶的不对称转化(由1控制,3-烯丙基型菌株)或主要由Ni0或RhI的手性络合物催化的炔烃的对映选择性[2 + 2 + 2]环异构化。有趣的是,形成了高级的螺旋结构,例如最长的螺旋(通过在一次合成操作中关闭12个环,最多达到oxa [19]螺旋)或横向延伸的螺旋(例如,pyreno [7]螺旋)。利用上述合成方法,可以更好地利用量身定制的螺旋分子纳米碳,用于对映选择性催化,手性传感,自旋电子学(基于手性引起的自旋选择性),手性学(产生圆偏振光发射),有机/分子电子或手性单分子设备。形成了先进的螺旋结构,例如最长的螺旋烯(通过在一次合成操作中关闭12个环,最多达到oxa [19]螺旋烯)或横向延伸的螺旋烯(例如,pyreno [7]螺旋烯)。利用上述合成方法,可以更好地利用量身定制的螺旋分子纳米碳,用于对映选择性催化,手性传感,自旋电子学(基于手性引起的自旋选择性),手性学(产生圆偏振光发射),有机/分子电子或手性单分子设备。形成了先进的螺旋结构,例如最长的螺旋烯(通过在一次合成操作中关闭12个环,最多达到oxa [19]螺旋烯)或横向延伸的螺旋烯(例如,pyreno [7]螺旋烯)。利用上述合成方法,可以更好地利用量身定制的螺旋分子纳米碳,用于对映选择性催化,手性传感,自旋电子学(基于手性引起的自旋选择性),手性学(产生圆偏振光发射),有机/分子电子或手性单分子设备。



























 京公网安备 11010802027423号
京公网安备 11010802027423号