当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interplay between Ionization and Tautomerism in Bioactive β-Enamino Ester-Containing Cyclic Compounds: Study of Annulated 1,2,3,6-Tetrahydroazocine Derivatives.
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jpcb.9b08904 Antonio Viayna 1 , Salvatore G Antermite 2 , Modesto de Candia 2 , Cosimo D Altomare 2 , F Javier Luque 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jpcb.9b08904 Antonio Viayna 1 , Salvatore G Antermite 2 , Modesto de Candia 2 , Cosimo D Altomare 2 , F Javier Luque 1
Affiliation
|
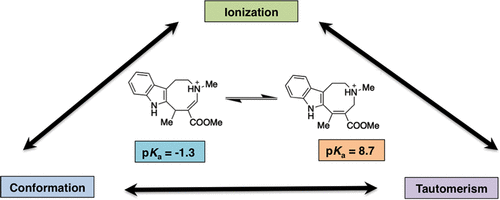
Depending on the chemical scaffold, a bioactive species could reflect the interplay between ionization and tautomerism, which is often complicated by the possibility of populating different conformational states, in the case of flexible ligands. In this context, theoretical methods can be valuable to discern the role of these factors, as shown here for β-enamino esters of 1,2,3,6-tetrahydroazocino-fused ring systems, some of which had proven to be suitable scaffolds for designing novel acetylcholinesterase inhibitors. The compounds investigated herein form two clusters with distinctive experimental pKa values (i.e., α,β-diesters and β-esters ranging within 6.1-7.3 and 8.2-9.0 pKa intervals, respectively), which implies a drastic difference in the most populated species at physiological conditions. While chemoinformatic tools did not provide a consistent description of the actual pKa values, the theoretical analysis performed for the protonated and neutral species of these compounds revealed a marked change in the tautomeric preference of the tetrahydroazocine moiety upon (de)protonation. Excellent agreement between the calculated and experimental pKa values was found when the tautomeric preference of the protonated and neutral species was considered. Overall, this study highlights the potential use of high-level computational methods to disclose the mutual influence between ionization, tautomerism, and conformational preferences in multifunctional (bio)organic compounds.
中文翻译:

含生物活性β-烯胺酯的环状化合物中电离和互变异构之间的相互作用:1,2,3,6-四氢偶氮azo嗪衍生物的研究。
根据化学支架的不同,生物活性物质可能反映出电离和互变异构之间的相互作用,在柔性配体的情况下,由于可能存在不同的构象状态,这种情况通常会变得复杂。在这种情况下,理论方法对于辨别这些因素的作用可能是有价值的,如此处所示的1,2,3,6-四氢偶氮芥子稠合环系统的β-烯胺酯,其中某些已被证明是适用于以下目的的支架设计新型乙酰胆碱酯酶抑制剂。本文研究的化合物形成两个簇,具有不同的实验pKa值(即,分别在6.1-7.3和8.2-9.0 pKa区间内的α,β-二酯和β-酯),这意味着在该温度下居于人口最多的物种之间存在巨大差异。生理条件。尽管化学信息学工具并未提供对实际pKa值的一致描述,但对这些化合物的质子化和中性物质进行的理论分析显示,四氢偶氮嗪部分的互变异构优先级在(去质子化)时发生了显着变化。当考虑质子化和中性物质的互变异构偏好时,发现在计算的pKa值和实验的pKa值之间存在极好的一致性。总的来说,这项研究强调了潜在的高级计算方法的使用,以揭示多功能(生物)有机化合物中电离,互变异构和构象偏好之间的相互影响。对这些化合物的质子化和中性物质进行的理论分析表明,(脱)质子化后,四氢偶氮嗪部分的互变异构优先级发生了显着变化。当考虑质子化和中性物质的互变异构偏好时,发现在计算的pKa值和实验的pKa值之间存在极好的一致性。总的来说,这项研究强调了潜在的高级计算方法的使用,以揭示多功能(生物)有机化合物中电离,互变异构和构象偏好之间的相互影响。对这些化合物的质子化和中性物质进行的理论分析表明,(脱)质子化后,四氢偶氮嗪部分的互变异构优先级发生了显着变化。当考虑质子化和中性物质的互变异构偏好时,发现在计算的pKa值和实验的pKa值之间存在极好的一致性。总的来说,这项研究强调了潜在的高级计算方法的使用,以揭示多功能(生物)有机化合物中电离,互变异构和构象偏好之间的相互影响。
更新日期:2019-12-29
中文翻译:

含生物活性β-烯胺酯的环状化合物中电离和互变异构之间的相互作用:1,2,3,6-四氢偶氮azo嗪衍生物的研究。
根据化学支架的不同,生物活性物质可能反映出电离和互变异构之间的相互作用,在柔性配体的情况下,由于可能存在不同的构象状态,这种情况通常会变得复杂。在这种情况下,理论方法对于辨别这些因素的作用可能是有价值的,如此处所示的1,2,3,6-四氢偶氮芥子稠合环系统的β-烯胺酯,其中某些已被证明是适用于以下目的的支架设计新型乙酰胆碱酯酶抑制剂。本文研究的化合物形成两个簇,具有不同的实验pKa值(即,分别在6.1-7.3和8.2-9.0 pKa区间内的α,β-二酯和β-酯),这意味着在该温度下居于人口最多的物种之间存在巨大差异。生理条件。尽管化学信息学工具并未提供对实际pKa值的一致描述,但对这些化合物的质子化和中性物质进行的理论分析显示,四氢偶氮嗪部分的互变异构优先级在(去质子化)时发生了显着变化。当考虑质子化和中性物质的互变异构偏好时,发现在计算的pKa值和实验的pKa值之间存在极好的一致性。总的来说,这项研究强调了潜在的高级计算方法的使用,以揭示多功能(生物)有机化合物中电离,互变异构和构象偏好之间的相互影响。对这些化合物的质子化和中性物质进行的理论分析表明,(脱)质子化后,四氢偶氮嗪部分的互变异构优先级发生了显着变化。当考虑质子化和中性物质的互变异构偏好时,发现在计算的pKa值和实验的pKa值之间存在极好的一致性。总的来说,这项研究强调了潜在的高级计算方法的使用,以揭示多功能(生物)有机化合物中电离,互变异构和构象偏好之间的相互影响。对这些化合物的质子化和中性物质进行的理论分析表明,(脱)质子化后,四氢偶氮嗪部分的互变异构优先级发生了显着变化。当考虑质子化和中性物质的互变异构偏好时,发现在计算的pKa值和实验的pKa值之间存在极好的一致性。总的来说,这项研究强调了潜在的高级计算方法的使用,以揭示多功能(生物)有机化合物中电离,互变异构和构象偏好之间的相互影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号