当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into structural and physicochemical properties required for β-hematin inhibition of privileged triarylimidazoles
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2019/12/16 , DOI: 10.1039/c9md00468h Clinton G. L. Veale 1, 2, 3, 4, 5 , Janeeka Jayram 1, 2, 3, 4, 5 , Shivani Naidoo 1, 2, 3, 4, 5 , Dustin Laming 5, 6, 7, 8 , Tarryn Swart 5, 6, 7, 8 , Tania Olivier 5, 9, 10, 11 , Matthew P. Akerman 1, 2, 3, 4, 5 , Katherine A. de Villiers 5, 9, 10, 11 , Heinrich C. Hoppe 5, 6, 7, 8 , Vineet Jeena 1, 2, 3, 4, 5
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2019/12/16 , DOI: 10.1039/c9md00468h Clinton G. L. Veale 1, 2, 3, 4, 5 , Janeeka Jayram 1, 2, 3, 4, 5 , Shivani Naidoo 1, 2, 3, 4, 5 , Dustin Laming 5, 6, 7, 8 , Tarryn Swart 5, 6, 7, 8 , Tania Olivier 5, 9, 10, 11 , Matthew P. Akerman 1, 2, 3, 4, 5 , Katherine A. de Villiers 5, 9, 10, 11 , Heinrich C. Hoppe 5, 6, 7, 8 , Vineet Jeena 1, 2, 3, 4, 5
Affiliation
|
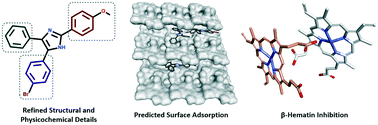
In this study, we investigated a series of triarylimidazoles, in an effort to elucidate critical SAR information pertaining to their anti-plasmodial and β-hematin inhibitory activity. Our results showed that in addition to the positional effects of ring substitution, subtle changes to lipophilicity and imidazole ionisability were important factors in SAR interpretation. Finally, in silico adsorption analysis indicated that these compounds exert their effect by inhibiting β-hematin crystal growth at the fast growing 001 face.
中文翻译:

深入了解β-血红素抑制特权三芳基咪唑所需的结构和物理化学性质
在这项研究中,我们研究了一系列三芳基咪唑,以阐明有关其抗疟原虫和β-血红素抑制活性的关键SAR信息。我们的结果表明,除了环取代的位置效应之外,亲脂性和咪唑离子化性的细微变化也是SAR解释的重要因素。最后,计算机吸附分析表明,这些化合物通过抑制快速增长的001面的β-hematin晶体生长发挥其作用。
更新日期:2020-02-13
中文翻译:

深入了解β-血红素抑制特权三芳基咪唑所需的结构和物理化学性质
在这项研究中,我们研究了一系列三芳基咪唑,以阐明有关其抗疟原虫和β-血红素抑制活性的关键SAR信息。我们的结果表明,除了环取代的位置效应之外,亲脂性和咪唑离子化性的细微变化也是SAR解释的重要因素。最后,计算机吸附分析表明,这些化合物通过抑制快速增长的001面的β-hematin晶体生长发挥其作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号